Computational Fluorescence Imaging
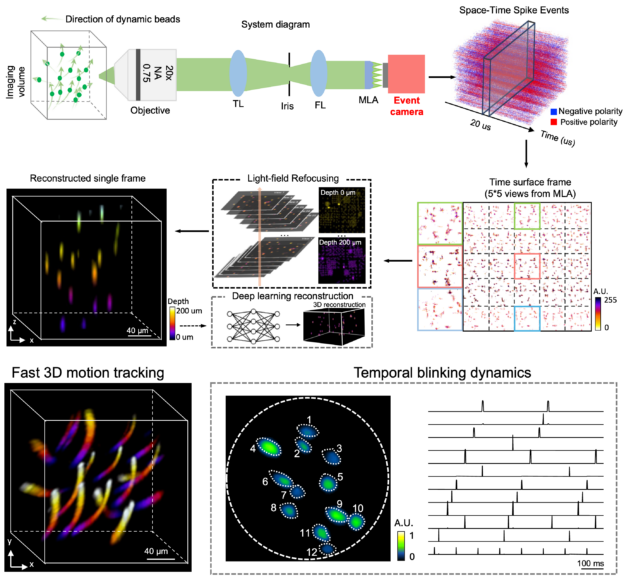
EventLFM: event camera integrated Fourier light field microscopy for ultrafast 3D imaging
Ruipeng Guo, Qianwan Yang, Andrew S. Chang, Guorong Hu, Joseph Greene, Christopher V. Gabel, Sixian You & Lei Tian
Light: Science & Applications 13: 144 (2024).
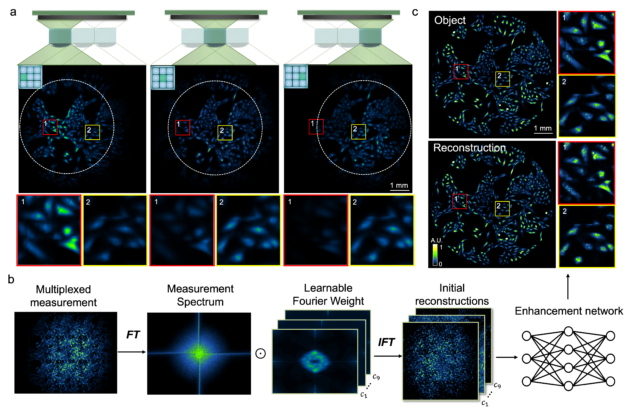
Wide-field, high-resolution reconstruction in computational multi-aperture miniscope using a Fourier neural network
Qianwan Yang, Ruipeng Guo, Guorong Hu, Yujia Xue, Yunzhe Li, and Lei Tian
Optica Vol. 11, Issue 6, pp. 860-871 (2024).
⭑ Github Project
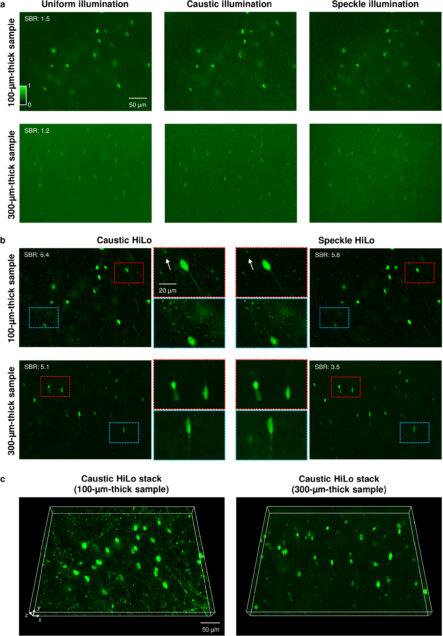
HiLo microscopy with caustic illumination
Guorong Hu, Joseph Greene, Jiabei Zhu, Qianwan Yang, Shuqi Zheng, Yunzhe Li, Jeffrey Alido, Ruipeng Guo, Jerome Mertz, and Lei Tian
Biomedical Optics Express Vol. 15, Issue 7, pp. 4101-4110 (2024).
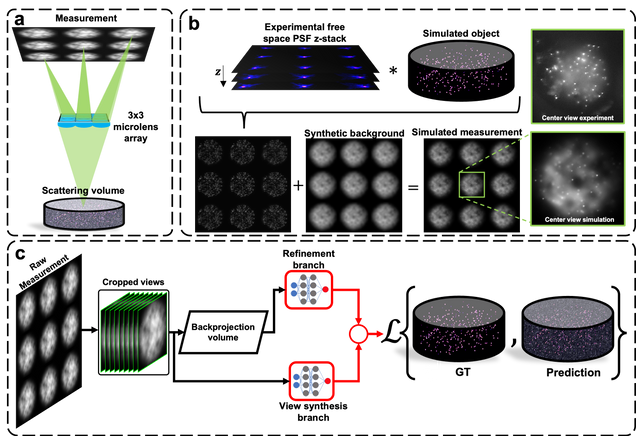
Robust single-shot 3D fluorescence imaging in scattering media with a simulator-trained neural network
J. Alido, J. Greene, Y. Xue, G. Hu, Y. Li, K. Monk, B. DeBenedicts, I. Davison, L. Tian
Optics Express Vol. 32, Issue 4, pp. 6241-6257 (2024).
⭑ Github Project
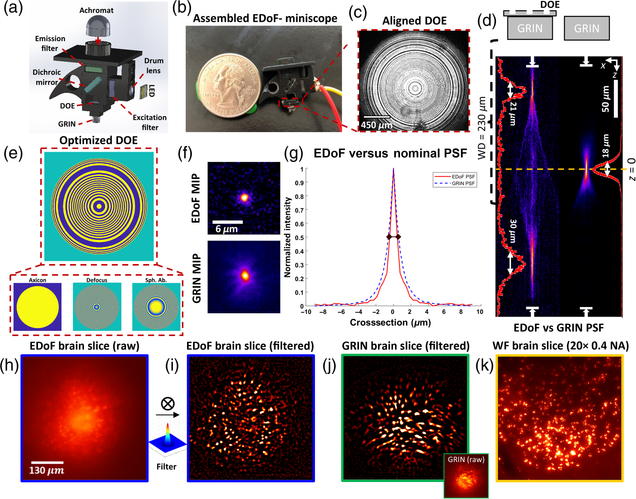
Pupil engineering for extended depth-of-field imaging in a fluorescence miniscope
Joseph Greene, Yujia Xue, Jeffrey Alido, Alex Matlock, Guorong Hu, Kivilcim Kiliç, Ian Davison, Lei Tian
Neurophotonics, Vol. 10, Issue 4, 044302 (2023).
Fluorescence head-mounted microscopes, i.e., miniscopes, have emerged as powerful tools to analyze in-vivo neural populations but exhibit a limited depth-of-field (DoF) due to the use of high numerical aperture (NA) gradient refractive index (GRIN) objective lenses. We present extended depth-of-field (EDoF) miniscope, which integrates an optimized thin and lightweight binary diffractive optical element (DOE) onto the GRIN lens of a miniscope to extend the DoF by 2.8 × between twin foci in fixed scattering samples. We use a genetic algorithm that considers the GRIN lens’ aberration and intensity loss from scattering in a Fourier optics-forward model to optimize a DOE and manufacture the DOE through single-step photolithography. We integrate the DOE into EDoF-Miniscope with a lateral accuracy of 70 μm to produce high-contrast signals without compromising the speed, spatial resolution, size, or weight. We characterize the performance of EDoF-Miniscope across 5- and 10-μm fluorescent beads embedded in scattering phantoms and demonstrate that EDoF-Miniscope facilitates deeper interrogations of neuronal populations in a 100-μm-thick mouse brain sample and vessels in a whole mouse brain sample. Built from off-the-shelf components and augmented by a customizable DOE, we expect that this low-cost EDoF-Miniscope may find utility in a wide range of neural recording applications.
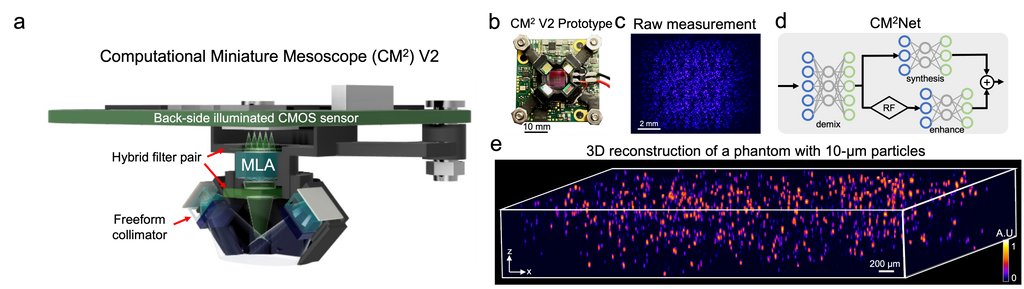
Deep learning-augmented Computational Miniature Mesoscope
Yujia Xue, Qianwan Yang, Guorong Hu, Kehan Guo, Lei Tian
Optica 9, 1009-1021 (2022)
Fluorescence microscopy is essential to study biological structures and dynamics. However, existing systems suffer from a tradeoff between field-of-view (FOV), resolution, and complexity, and thus cannot fulfill the emerging need of miniaturized platforms providing micron-scale resolution across centimeter-scale FOVs. To overcome this challenge, we developed Computational Miniature Mesoscope (CM
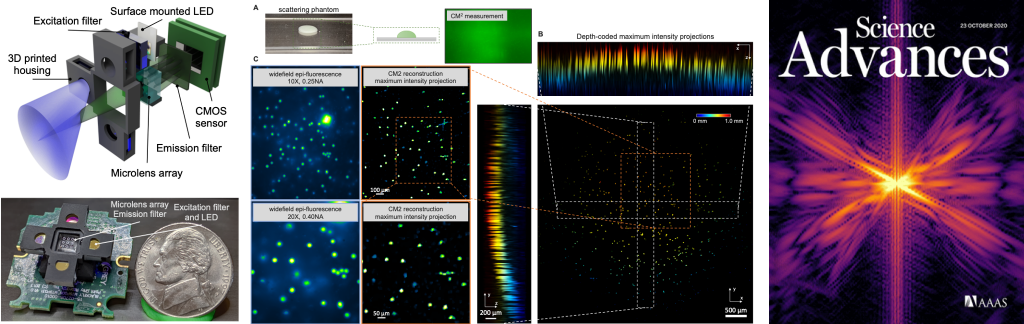
Single-Shot 3D Widefield Fluorescence Imaging with a Computational Miniature Mesoscope
Yujia Xue, Ian G. Davison, David A. Boas, Lei Tian
Science Advances 21 OCT 2020: EABB7508
⭑ On the Cover
⭑ In the news:
– BU ENG news: Brain Imaging Scaled Down
Fluorescence microscopes are indispensable to biology and neuroscience. The need for recording in freely behaving animals has further driven the development in miniaturized microscopes (miniscopes). However, conventional microscopes/miniscopes are inherently constrained by their limited space-bandwidth product, shallow depth of field (DOF), and inability to resolve three-dimensional (3D) distributed emitters. Here, we present a Computational Miniature Mesoscope (CM2) that overcomes these bottlenecks and enables single-shot 3D imaging across an 8 mm by 7 mm field of view and 2.5-mm DOF, achieving 7-μm lateral resolution and better than 200-μm axial resolution. The CM2 features a compact lightweight design that integrates a microlens array for imaging and a light-emitting diode array for excitation. Its expanded imaging capability is enabled by computational imaging that augments the optics by algorithms. We experimentally validate the mesoscopic imaging capability on 3D fluorescent samples. We further quantify the effects of scattering and background fluorescence on phantom experiments.
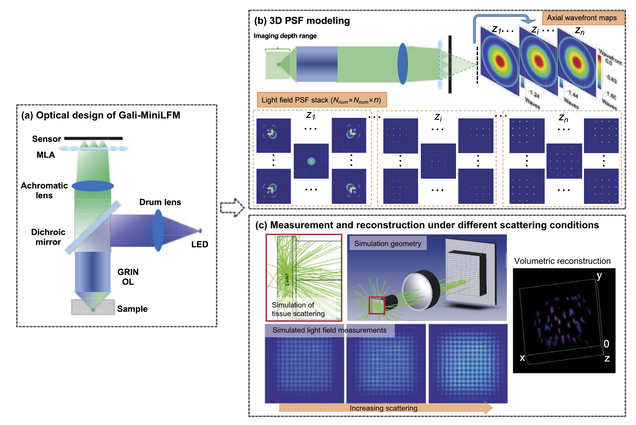
Design of a high-resolution light field miniscope for volumetric imaging in scattering tissue
Yanqin Chen, Bo Xiong, Yujia Xue, Xin Jin, Joseph Greene, and Lei Tian
Biomedical Optics Express 11, pp. 1662-1678 (2020).
Integrating light field microscopy techniques with existing miniscope architectures has allowed for volumetric imaging of targeted brain regions in freely moving animals. However, the current design of light field miniscopes is limited by non-uniform resolution and long imaging path length. In an effort to overcome these limitations, this paper proposes an optimized Galilean-mode light field miniscope (Gali-MiniLFM), which achieves a more consistent resolution and a significantly shorter imaging path than its conventional counterparts. In addition, this paper provides a novel framework that incorporates the anticipated aberrations of the proposed Gali-MiniLFM into the point spread function (PSF) modeling. This more accurate PSF model can then be used in 3D reconstruction algorithms to further improve the resolution of the platform. Volumetric imaging in the brain necessitates the consideration of the effects of scattering. We conduct Monte Carlo simulations to demonstrate the robustness of the proposed Gali-MiniLFM for volumetric imaging in scattering tissue.