URBAN ARCH Expands Sample Collection and Assessment Methods
At the core of URBAN ARCH research is the data that is collected for analysis and repository storage. Across the consortium, research assessors are implementing new methods for participant assessment and biological sample collection. These innovative techniques are allowing robust data collection to quantify participants’ substance use, physical health, and pain. In this issue, we take a close look at the latest sample collection and participant assessment techniques that researchers are using to gather data in Boston, Russia, and Uganda.
Boston
The Boston ARCH 4F study is testing the associations between alcohol consumption and falls and fractures. To assess participants’ frailty and potential susceptibility to falls and fractures, RAs measure participants’ grip strength using a hand dynamometer. With their elbow resting against their side, the participant grips the handle as hard as they can 6 times total, 3 with each hand.
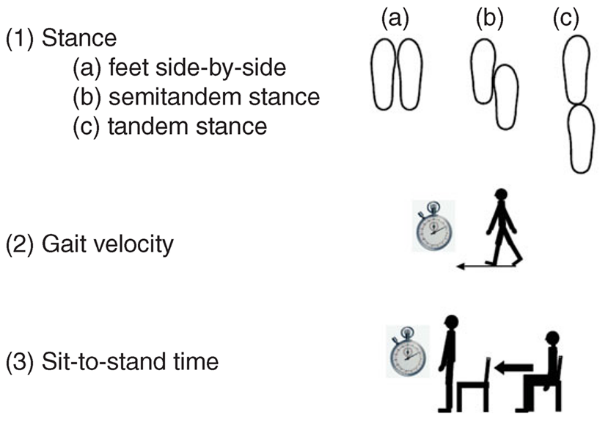
Next, researchers put participants through the Short Physical Performance Battery (SPPB) to assess their balance, agility, and strength (see image). They begin with balance tests: side-by-side stand (standing with feet together, side-by-side for 30 seconds); semi-tandem stand (standing with the side of the heel of one foot touching the big toe of the other foot for around 30 seconds); tandem stand (standing with the heel of one foot in front of and touching the toes of the other foot for about 30 seconds); single leg stance (standing on one leg for as long as they can, up to 30 seconds). Then participants are observed and timed walking through a course, first for 4 meters, then for 20, if they complete 4 meters without trouble. Then they are asked to repeat the 20 meter walk, if they successfully completed the first. The final test requires the participant to get up from a chair with their arms crossed over their chest. If the first attempt is successful, they are then asked to repeat it 10 times, while being timed by the researcher.
Researchers are also collecting samples of participants’ saliva at baseline and annually. These samples are stored for future testing in the URBAN ARCH Data and Sample Repository. Research assistants use micro-centrifuge tubes to collect 0.75mL – 1.2mL of saliva, with any sample over 1mL considered to be “full.” Participants cannot eat, drink, smoke, or use oral hygiene products for 30 minutes prior to the collection. The saliva samples must be processed and frozen immediately.
Russia

Participants in the PETER PAIN study have literally been trying their hand at research in the study’s cold-presser machine (See photo). To assess whether study medications have an effect on participants’ pain tolerance, an ice/water bath is prepared to the temperature of 0.5-1 degree C. The participant then places their hand into the water up to the wrist, leaving it there for as long as they can tolerate it. Participants are asked to notify assessor when they first feel pain. Using a stopwatch, assessor also notes the time at which the participant removes their hand from the machine. After 3 minutes, participants are asked to remove their hands from the machine if they have not done so themselves already. They are then asked to rate the severity of the pain they experienced on a scale of 0-100.
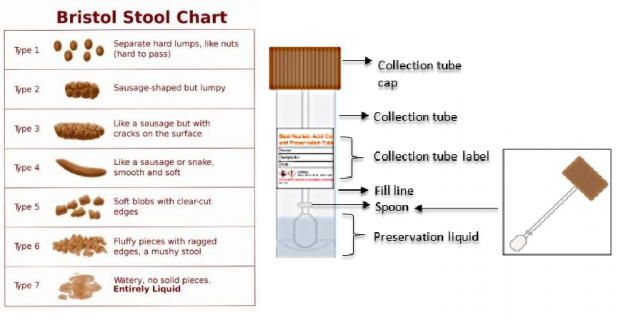
Researchers conducting the ACME HIV study are recruiting up to 200 participants from the St PETER HIV and TMAO studies for an observational cohort study. Participants have HIV and heavy alcohol use. Data to be collected includes fecal sample (12-months), Bristol Stool Scale (12-months), , and gut microbiome metagenomic sequencing data (baseline, 3-, and 12-months).
The fecal sample testing will provide information on how heavy alcohol use affects the structure and function of gut microbial communities. It will be collected at baseline and 3-month time points along with the 12-month sample collected in ACME HIV. Participants take the sample collection kit home with them and give it to the study staff when they return for study appointments. At the times of collection, participants are given the Bristol Stool Chart (see image) and asked to characterize their stool at the time of collection.
If participants are performing the sample collection at home, they are asked to make the collection 0-2 days before their next visit. They are given nitrile gloves, special paper to assist with collection, and a sample collection tube (see figure) to ensure the integrity of the sample collection.
Fecal samples are stored at the First St. Petersburg Pavlov State Medical University (PSMU) in St. Petersburg, Russia; they are sent to the United States in batches for testing.

ACME HIV: Lung Microbiome is testing the effects that alcohol and tobacco consumption have on the bacteria in participants’ lungs. The study is an observational ancillary study of up to 150 people with HIV nested in the ongoing ACME HIV observational study of 200 people with HIV. To test the lung microbiome, nasal secretion samples will be collected at 2-weeks, 3-months, and 12-months. At sample collection, specimens are collected from each nostril by using sterilized tweezers to place a paper filter in each nostril for two minutes (see photo) and then placing the papers into separate sterile collection tubes. The samples are then immediately frozen before being sent to the US for testing. Testing of the nasal samples will take place at Vanderbilt University Medical Center and will include 16s rRNA sequencing. Resulting 16s data will be combined and analyzed with existing data from the St PETER HIV, TMAO, and ACME HIV studies to understand if changes in tobacco and alcohol consumption impact the lung microbiome and how changes in the lung microbiome are related to other health measures such as the gut microbiome.
Uganda
URBAN ARCH researchers working on the Alcohol Drinkers’ Exposure to Preventive Therapy for Tuberculosis (ADEPTT) study in Uganda have turned to hair samples to test levels of isoniazid among participants (see photo). Eligible study participants have HIV and latent TB— some with alcohol use and some without. Testing the isoniazid levels in the hair samples will help researchers understand TB treatment safety, efficacy, and adherence among patients with and without alcohol use. Hair samples must be handled carefully, kept at room temperature and in the dark prior to shipment to UCSF’s hair laboratory for testing. Any hair that remains after testing will be stored in the URBAN ARCH repository at Boston University.