International URBAN ARCH Center Core and Project Updates
Administrative Core
The Admin Core successfully planned and coordinated the 2024 URBAN ARCH Annual Meeting on May 3, 2024. You can read about the meeting in this issue’s main story. Of note, we were able to spotlight four early stage investigator presentations at this year’s meeting, as well as a keynote presentation by Dr. Bob Horsburgh on current issues in HIV and TB research.
In conjunction with this year’s RSA Satellite Meeting on HIV and Alcohol on June 22 in Minneapolis, MN, the Admin Core will coordinate one-on-one mentoring sessions for early career investigators.
The Core is currently preparing for another year of training and mentoring events. We plan to continue hosting quarterly Visiting Scholar Research-in-Progress webinars and bi-monthly Training and Mentoring Workshops, come September. Stay tuned for more information!
Biostatistics and Data Management Core
The BDM Core has been working with the project teams to advance the progress of both the TRAC and TALC studies. Data collection and data management activities are progressing smoothly for each study. For THAU 50/50, the REDCap build is complete and CRFs were formally tested in REDCap by BEDAC and the study team. Several manuscripts remain in progress, e.g. association between alcohol and inflammatory biomarkers; smoking cessation; oxidative stress; TMAO. The core has also been working to complete analyses and collaborating on several manuscripts that are in preparation.
TB Risk by Alcohol Consumption (TRAC) Study
The aims of the TB Risk by Alcohol Consumption (TRAC) study are to estimate the incidence rate of new TB infection among people with HIV (PWH) with prior negative tuberculin skin test (TST) results by level of alcohol use (Aim 1) and to determine the incidence of active TB disease among PWH with prior latent TB infection (LTBI), who received TB preventative therapy (TPT), by level of alcohol use (Aim 2).

As of May 31, 2024, we have successfully concluded TRAC study enrollment with 500 participants. To achieve this goal, we made phone call attempts to 694 prior study participants for Aim 1 screening. We reached 558 participants and invited all of them for screening. Of the 558 screened, we found 522 to be eligible, while 20 people declined consent to participate in the study. Therefore, we enrolled a total of 502 participants into the study, however 2 participants were enrolled in error and were subsequently excluded, leaving the total number enrolled as 500. Self-reported AUDIT-C scores from TRAC baselines visits show 138 (28%) in the no alcohol group, 203 (41%) in the low/moderate alcohol use group, and 158 (32%) in the high-risk alcohol use group. Baseline PEth results of 451 participants show 155 (34%) in the no alcohol use group with PEth levels of <20 ng/ml, 155 (34%) in the no alcohol use group with PEth levels of 20-<200 ng/ml, 155 (34%) in the no alcohol use group with PEth levels of >200 ng/ml.

All enrolled participants have completed baseline procedures including questionnaire, biological specimen collection for dried blood spot (DBS) preparation and storage for PEth testing, urine collection for real time cotinine testing, and PPD placement.
Of the 500 (217 (43%) females and 283 (57%) males) enrolled with a median age of 42 years, 75 had positive TST results following placement and reading of TST within 72 hours. After excluding 4 participants (2 with prior active TB on chart review, and 2 with no TST result), and including 8 with active TB on chart review but with TST negative results, we found 83 out of 496 (16.7%) had new TB infection/disease. We found no associations with active TB/positive TST at baseline by smoking status, household TB diagnosis, or number of rooms in the household.

We successfully completed 474 6-month follow up phone calls (95% completion rate), 388 12-month in-person visits (97% completion rate), 253 18-month follow-up phone calls (98% completion rate), and 58 24-month in-person visits (99% completion rate). At the 12- and 24-month visits, we placed PPDs on only those who were negative at baseline or 12 months. Of the 340 PPDs placed at the 12-month visit, 36 were positive (10% positivity rate). Of the 46 PPDs placed at the 24-month visit, 3 were positive (7% positivity rate). Seven participants were disenrolled from the study and five died due to reasons unrelated to study participation.
Aim 2 procedures are in progress. We have completed two rounds of review from TB registries, clinical registries, and medical records in 18 clinics from which participants were recruited in previous studies. We found all records (988 total) included in Aim 2. Of the 988 records reviewed, 29 (2.9%) participants were diagnosed with active TB after receiving INH and 31 (3.1%) participants were dead. We are investigating the cause of death in these participants. We also found that 103 (10.4%) participants transferred out of their original clinics.
Tuberculosis, Alcohol, and Lung Comorbidities (TALC) Study
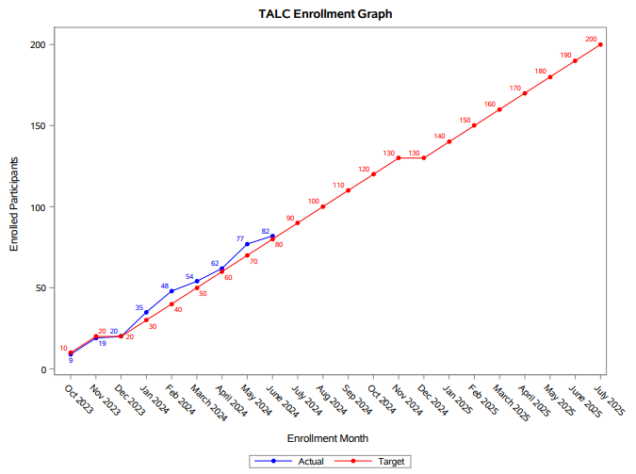
The TALC team continues to make progress on our enrollment goals, and we are nearing 50% enrollment. In Aim 1 of the study, we will enroll 200 participants in order to investigate the relationship between alcohol use and post-TB lung disease in people with HIV (PWH). The team has screened 89 participants for Aim 1, of which 82 are currently enrolled and have completed the baseline visit. The team is actively completing follow-up visits at the 3- and 6-month time points. The team will begin completing 9-month follow-up visits in July.
The team has also been working to obtain a spirometer with DLCO measurement capabilities for Aim 1 of the study. This equipment has been purchased and shipped to the airport in Uganda, and the team is currently working through the clearing process so that it can be transported to Mbarara. We are looking forward to receiving the equipment in Mbarara so that the team can integrate DLCO collection into follow-up visits going forward.

In Aim 2, we will qualitatively evaluate factors for tailoring alcohol and smoking interventions in PWH being treated for TB. To this end, we will interview 24 TALC study participants and 12 key informants, including TB providers and other health workers. As of June 13, 2024, the team has completed 7 interviews with participants and 8 interviews with providers.
On April 26, TALC site coordinator Naomi Sanyu presented at the Community Advisory Board (CAB) meeting in Mbarara. The CAB provides guidance for several study teams within the Global Health Collaborative at Mbarara University of Science and Technology (MUST), of which our study team is a part. The CAB provided valuable feedback on study activities and their impact on participants and the community.
All regulatory approvals have been received for the TB HIV Aging in Uganda 50-over-50 (THAU 50/50) sub-study. The team is currently in the process of hiring an additional RA for this sub-study, and developing training materials. We hope to begin enrollment this summer.
Gabapentin to Reduce Alcohol and Improve Viral Load Suppression (GRAIL) Study
GRAIL study (NIAAA R01) enrollment began in November 2023, and as of June 9, 2024, the study has enrolled and randomized 53/300 participants. A total of 181 pre-screenings and 170 in-person screenings have been completed to date. The main reasons for ineligibility include a detectable HIV viral load (42/170; 24.7%), a negative AUDIT-C score (27/170; 15.9%), and a negative EtG test (35/170; 20.6%). Women comprise 25% of the sample. The average age of enrolled participants is 39 years and 18 had an AUDIT score >=20. The study team is actively completing follow-up visits at weeks 1, 2, 3, 6, and 10, and months 1, 2, 3 (162 in-person and 158 phone visits completed to date) with follow-up rates above 80% for all visits. Recognizing the need to enhance recruitment, the team has recently extended recruitment efforts to additional sites and is actively preparing to expand to further sites.