4F
The 4F Study: A summary of the Boston ARCH renewal study, Alcohol and HIV-Associated Comorbidity and Complications:
Frailty, Functional Impairment, Falls, and Fractures (2016-2021)
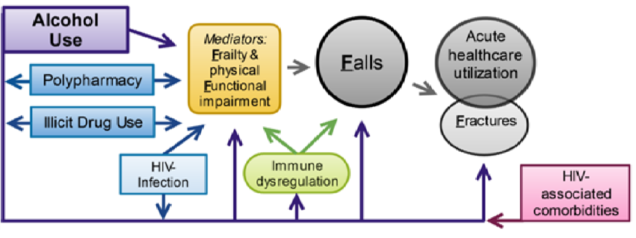
The Boston ARCH renewal study, Alcohol and HIV-Associated Comorbidity and Complications: Frailty, Functional Impairment, Falls, and Fractures (The 4F Study), will follow and expand the original cohort to test the association between alcohol (and illicit drugs and polypharmacy) and frailty, functional impairment, falls, and fractures. Up to 30% of people living with HIV (PLWH) experience falls each year, which can lead to fractures that are 40-60% more common in PLWH. Frailty occurs even among virally suppressed PLWH, and is associated with fractures, falls, and hospitalizations, leading to high medical costs and excess hospitalizations. Most existing fall prevention interventions do not address risk factors that are important for PLWH, such as alcohol and other drugs, HIV medications, or HIV complications. Interventions are therefore needed for PLWH, and cohort studies such as Boston ARCH will provide information to understand risk factors in order to develop targeted interventions.
The specific aims of the 4F Study are:
- To test the associations between alcohol (and illicit drug use and polypharmacy) and falls (fractures secondarily); and whether frailty mediates these associations.
- To test the associations between alcohol (and illicit drugs and polypharmacy) and utilization (emergency department use and hospitalization for falls and fractures); and whether frailty mediates them.
- (EXPLORATORY AIM) To develop, based on aims 1 and 2 findings, and pilot test the feasibility of a falls prevention intervention tailored to address the identified risk factors (e.g. alcohol, illicit drugs, polypharmacy) in this population.
The Boston ARCH cohort had a remarkable 98% retention rate in the first cycle (2011-2016). The 4F Study plan includes the following:
- Re-enrolling close to 250 participants from the original cohort and enrolling an additional 150 new participants
- Enrolling participants with any past 12 months use of illicit drugs, marijuana (not recommended by a healthcare provider), or nonmedical use of prescription medications OR past 12 month alcohol use with positive AUDIT-C score (>3 for females and >4 more males)
- An in-person interview every 12 months for up to 36 months, as well as 6-month interim telephone assessments
- Blood sample collection for the URBAN ARCH repository (plasma, serum, dried blood spots)
- Grip strength (dynamometer) and short physical performance battery assessments
- Self-reports using the NIAID AIDS Clinical Trials Group (ACTG) Fall History Questionnaire as well as self-reported recent emergency department visits and hospitalizations (these self-reports will be validated with electronic medical records and statewide utilization data where applicable)
l-r; Seville Meli, Theresa Kim, Rich Saitz, Mariana Krueger, Jasmin Choi, Aldina Mesic, and Susie Kim at a Boston ARCH meeting.
Study recruitment begins this Fall. The 4F Study has the potential to better understand risk factors in PLWH to develop interventions that will prevent falls.
Want to learn more about 4F? Click here for our July – September 2017 newsletter issue spotlight on Traci Green.
- Principal Investigator:
- Richard Saitz, MD, MPH
- Co-investigators:
- Traci Green, PhD, MSc
- Alan Jette, PT, PhD
- Meg Sullivan, MD
- Alexander Walley, MD, MSc
- Project Managers:
- Mariana Krueger, MS
- Seville Meli, MPH
- Research Assistants:
- Jasmin Choi, MSW
- Aldina Mesic, BA