The estimated annual financial impact of gene therapy in the United States
By Chi Heem Wong, Dexin Li, Nina Wang, Jonathan Gruber, Andrew W. Lo & Rena M. Conti
Gene therapy is a new class of medical treatment that alters part of a patient’s genome through the replacement, deletion, or insertion of genetic material. While still in its infancy, gene therapy has demonstrated immense potential to treat and even cure previously intractable diseases. Nevertheless, existing gene therapy prices are high, raising concerns about its affordability for U.S. payers and its availability to patients. We assess the potential financial impact of novel gene therapies by developing and implementing an original simulation model which entails the following steps: identifying the 109 late-stage gene therapy clinical trials underway before January 2020, estimating the prevalence and incidence of their corresponding diseases, applying a model of the increase in quality-adjusted life years for each therapy, and simulating the launch prices and expected spending of all available gene therapies annually (please see figures 5 and 6 below). The results of our simulation suggest that annual spending on gene therapies will be approximately $20.4 billion, under conservative assumptions. We decompose the estimated spending by treated age group as a proxy for insurance type, finding that approximately one-half of annual spending will on the use of gene therapies to treat non-Medicare-insured adults and children. We conduct multiple sensitivity analyses regarding our assumptions and model parameters. We conclude by considering the tradeoffs of different payment methods and policies that intend to ensure patient access to the expected benefits of gene therapy.
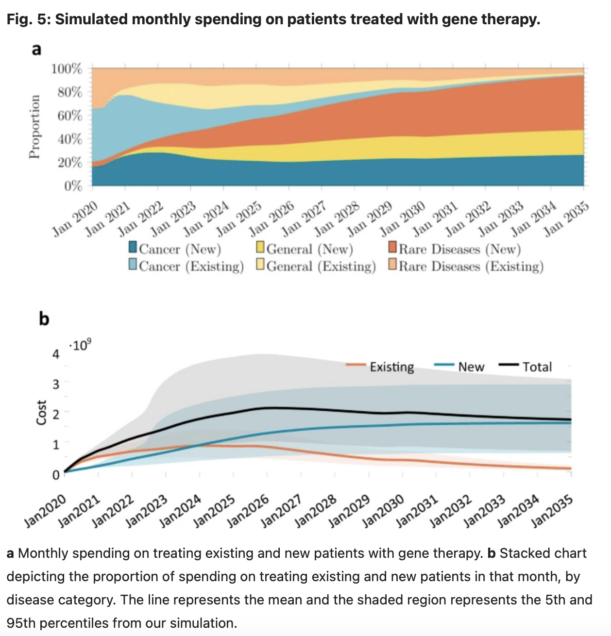
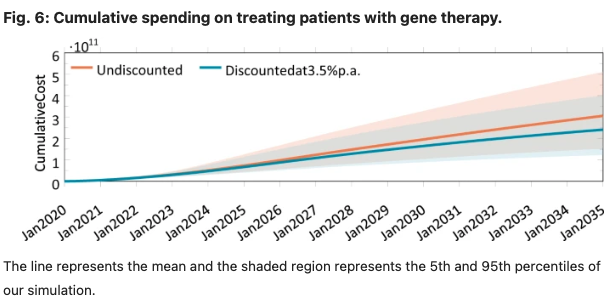
In this paper, we developed and implemented a novel mathematical model to estimate the expected annual number of patients treated with gene therapy over time, and the annual cost of gene therapy in the U.S. overall and by payer. It is our hope that this study, and our estimates of the potential financial impact of gene therapy in the U.S., will provide more clarity on the potential clinical and fiscal impacts of this new class of treatment and identify uncertainties involved in any projection of expected spending on these therapies in aggregate and by specific U.S. payers. We hope this work will help decision-makers, including patients, physicians, hospital administrators, health plans, employers, drug companies and other innovators, and policymakers make informed decisions about future access and reimbursement for this novel therapeutic class.