Category: Research
Funding
1R01DA039168-A1 (PI: Bryant) $3,026,929 04/01/2015-03/31/2020
NIH/NIDA: Bridging Genetic variation with Behavior: Molecular and Functional Mechanisms of Quantitative Trait Gene Regulation of the Stimulant and Addictive Properties of Methamphetamine in Mice
Use TALENs and CRISPR/Cas9 to make knockout and knockin mice carrying Hnrnph1 and Rufy1 to identify the quantitative trait gene influencing sensitivity to methamphetamine behaviors and use transcriptome analysis to identify the molecular mechanisms.
R00DA029635 (PI: Bryant) $737,472 05/01/2011-04/30/2016
NIH/NIDA: Genetic Basis of Opioid Reward and Aversion in Mice
Use QTL, transcriptome, and gene targeting approaches to identify the genetic basis and molecular mechanisms of the motivational properties of opioids in mice.
R00DA029635 05S1 (PI: Bryant) $7,926 06/01/2015-08/30/2015
NIH/NIDA: Genetic Basis of Opioid Reward and Aversion in Mice
Supplement to fund the stipend of a summer NIDA undergraduate student through their summer program.
R03DA038287 (PI: Bryant) $175,472 07/01/2014-06/30/2016
NIH/NIDA: Mapping G x E Interactions for Addiction Traits in a Reduced Complexity Cross
Map the genetic basis of gene by environment interactions in a panel of addiction traits using an F2 cross between C57BL/6 substrains and validate candidate genes using TALENs designer nucleases.
Spivack Award (PI: Bryant) $8,000 01/01/2015-12/31/2015
Spivack Scholars of Boston University 2014 Awards
Posters
Csnk1e project
Lisa Goldberg GPN recruitment poster
Olga Lacki Fall 2014 UROP poster
Food Addiction project
Stacey Kirkpatrick ACNP Poster
Rufy1 project
Camron Bryant WCPG Poster
Publications
Zhou L, Bryant CD, Loudon AS, Palmer AA, Vitaterna MH, Turek FW (2014). The circadian clock gene Csnk1e regulates REM sleep and NREM sleep architecture in mice. In press, Sleep.
Bryant CD, Guido MA, Kole LA, Cheng R. The heritability of oxycodone reward and concomitant phenotypes in a LG/J x SM/J F48 mouse advanced intercross line (2013). Addiction Biology, Dec 12. doi: 10.1111/adb.12016. [Epub ahead of print]
Bryant CD, Kole LA, Guido MA, Cheng R, Palmer AA (2012). Methamphetamine-induced conditioned place preference in LG/J and SM/J mouse strains and an F45/F46 advanced intercross line. Frontiers in Genetics 3:126
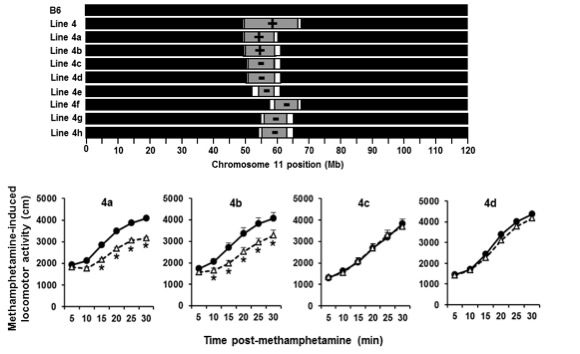
Bryant CD, Kole LA, Guido MA, Sokoloff G, Palmer AA (2012). Congenic dissection of a major QTL for methamphetamine sensitivity implicates epistasis. Genes, Brain and Behavior 11(5):623-32
Bryant CD, Parker CC, Zhou L, Olker C, Chandrasekaran RY, Wager TT, Bolivar VJ, Loudon AS, Vitaterna MH, Turek FW, Palmer AA (2012). Csnk1e is a genetic regulator of sensitivity to psychostimulants and opioids. Neuropsychopharmacology 37(4):1026-35.
Bryant CD (2011). The blessings and curses of C57BL/6 substrains in mouse genetic studies. Annals of the New York Academy of Sciences 1245(1):31-3.
Bryant CD, Chang HP, Zhang J, Wiltshire T, Tarantino LM, Palmer AA (2009). A major QTL on chromosome 11 influences psychostimulant and opioid sensitivity in mice. Genes, Brain and Behavior 8(8):795-805.
Hofford RS, Hodgson SR, Roberts KW, Bryant CD, Evans CJ, Eitan S (2009). Extracellular signal-regulated kinase activation in the amygdala mediates elevated plus maze behavior during opioid withdrawal. Behavioural Pharmacology 20(7):576-583.
Bryant CD, Roberts KW, Culbertson CS, Le A, Evans CJ, Fanselow MS (2009). Pavlovian conditioning of multiple opioid-like responses in mice. Drug and Alcohol Dependence 103:74-83.
Bryant CD, Graham ME, Distler MG, Munoz MB, Li D, Vezina P, Sokoloff G, Palmer AA (2009). A role for casein kinase 1 epsilon in the locomotor stimulant response to methamphetamine. Psychopharmacology 203(4):703-11.
Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, McRoberts JA (2008). Behavioral differences among C57BL/6 substrains: Implications for transgenic and knockout studies. Journal of Neurogenetics 22(4):315-31.
Gioiosa L, Chen X, Watkins R, Klanfer N, Bryant CD, Evans CJ, Arnold AP (2008). Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Hormones and Behavior 53(1):124-130.
Bryant CD, Roberts KW, Byun JS, Fanselow, MS, Evans CJ (2006). Morphine analgesic tolerance in 129P3/J and 129S6/SvEv mice. Pharmacology, Biochemistry and Behavior 85(4):769-79.
Bryant CD, Eitan S, Sinchak K, Fanselow MS, Evans CJ (2006). NMDA receptor antagonism disrupts the development of morphine analgesic tolerance in male, but not female C57BL/6J mice. American Journal of Physiology: Regulatory, Comparative and Integrative Physiology 292(2):R315-R326.
Bryant CD, Zaki PA, Carroll FI, Evans CJ (2005). Opioids and Addiction: Emerging pharmaceutical strategies for reducing reward and opponent processes. Clinical Neuroscience Research 5:103-115. Review.
Lutfy K, Eitan S, Bryant CD, Yang YC, Walwyn W, Saliminejad N, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, and Evans CJ (2003). Buprenorphine-induced antinociception is mediated by mu receptors and compromised by concomitant activation of opioid-like receptor-1 receptors. Journal of Neuroscience 23(32):10331-7.
Eitan S, Bryant CD, Saliminejad N, Yang YC, Vojdani E, Polakiewicz R, Evans CJ (2003). Brain region-specific mechanisms of morphine-induced MAPK modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. Journal of Neuroscience 23(23):8360-9.
Wilson SG*, Bryant CD*, Lariviere WR, Olsen MS, Giles BE, Chesler EJ, Mogil JS (2003). The heritability of antinociception II: Pharmacogenetic mediation of three over-the-counter analgesics in mice. Journal of Pharmacology and Experimental Therapeutics 305(2):755-64.
Genetic basis of the placebo response
Dr. Bryant has a longstanding interest in using mice to study the neurobiological basis of the “placebo effect” (Bryant et al., 2009; Drug and Alcohol Dependence), a phenomenon that has been hypothesized to be mediated by the reward expectation. He plans to develop and apply a forward genetic analysis toward Pavlovian conditioning mouse models across a variety of conditions that are notoriously sensitive to the placebo effect, including pain, anxiety, depression, and Parkinson’s Disease.
Candidate gene testing of Rufy1 and Hnrnph1 for methamphetamine sensitivity
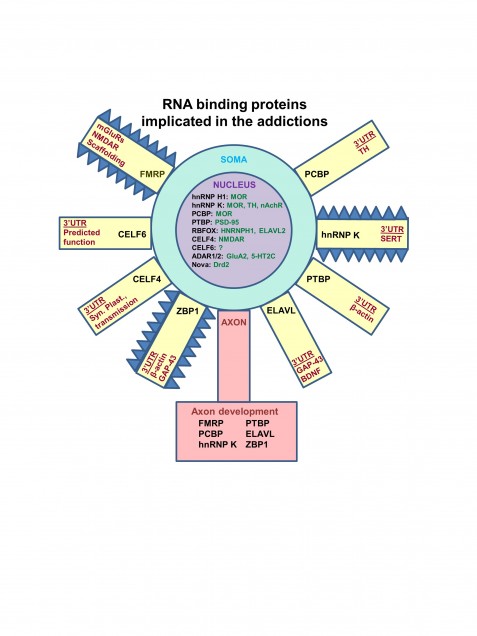
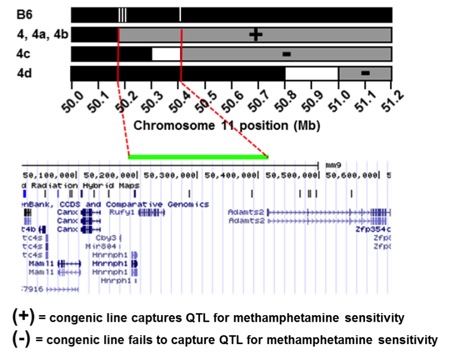
Dr. Bryant recently generated a panel of 17 interval-specific congenic mice to identify a 0.25 Mb locus on chromosome 11 that regulates the locomotor stimulant response to methamphetamine (MA) (manuscript in preparation). This genomic region contains only two possible quantitative trait genes that could be responsible for the QTL: Rufy1 and Hnrnph1. Rufy1, also known as Rabip4, codes for an early endosomal protein that interacts with Rab proteins including Rab4 and Rab5. We hypothesize that a known coding polymorphism within Rufy1 alters methamphetamine-induced trafficking of its primary molecular targets – the monoamine transporters – which in turn affects dopamine release, uptake, and behavior. We are directly targeting Rufy1 in vivo using TALENs technology and will directly test the role of Rufy1 in methamphetamine-induced transporter trafficking, dopamine uptake/release, and behavior. We are also targeting the second gene within the QTL interval, Hnrnph1, and examining its effect on gene transcription and behavior. Hnrnph1 is a heterogenous nuclear ribonucleoprotein that has been shown to regulate processing of microRNAs (miRNAs) and thus, could affect the expression of gene networks. The results of these studies will lead to the identification of the quantitative trait gene (QTG) that underlies the QTL responsible for genetic variation in methamphetamine sensitivity. Future directions will include examining this QTG in other conditions that could potentially be affected by dopaminergic function (Parkinson’s, ADHD, schizophrenia) and determining its role in regulating the motivational responses to methamphetamine and other substances of abuse.
Deciphering the roles of casein kinase 1-epsilon and –delta in the motivational properties of substances of abuse and dopaminergic signaling.
Dr. Bryant recently provided direct genetic and pharmacological evidence that casein kinase 1-epsilon (Csnk1e) inhibits whereas casein kinase 1-delta (Csnk1d) facilitates the locomotor response to psychostimulants and opioids (Bryant et al., 2012; Neuropsychopharmacology). These observations suggest at least two possibilities regarding the molecular and cellular mechanisms of these two highly related isoforms on behavior: 1) Csnk1e and Csnk1d modulate different signaling pathways to produce their opposing behavioral effects and 2) Csnk1e and Csnk1d are expressed in different cell types and thus, modulate different circuitry to exert opposing effects on behavior. We are currently testing these two hypotheses by examining dopamine signaling (DARPP-32) and generating cell type-specific knockout mice to examine the effects of ablating Csnk1e versus Csnk1d on signaling and behavior.
Lisa Goldberg Casein Kinase-1 Deletion poster
Olga Lacki Fall 2014 UROP poster
K99/R00: “Genetic basis of opioid reward and aversion in mice.”
A current focus is to determine the genetic basis of the rewarding properties of opioids in mice by combining quantitative trait locus (QTL) analysis of conditioned place preference (CPP) and transcriptome analysis via RNA-sequencing in genetic reference populations that yield high resolution QTLs. This multi-pronged approach to gene mapping will accelerate the nomination of candidate genes for validation via direct gene targeting. We are dissecting multiple behaviors that are expressed during opioid administration and CPP in real time (locomotor, visits, visit time, ultrasonic vocalizations, placebo-like responses) and applying factor analysis toward defining behavioral sets and inferring motivational meaning of these factors. We will estimate heritability for these trait sets and apply QTL mapping toward those most influenced by genetic factors. Elucidating the genetic basis of the motivational properties of opioids and other drugs of abuse will have direct application toward preventative/treatment strategies of addiction in humans, toward other substances of abuse (ethanol and food), and toward other conditions affected by the mesocorticolimbic pathway (Parkinson’s, depression, schizophrenia).