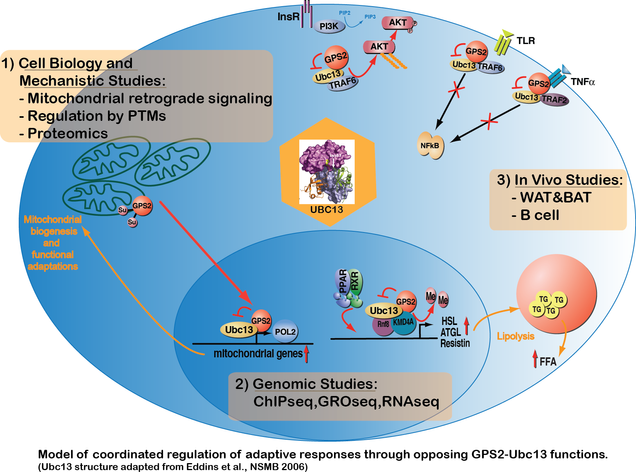
The overarching theme in the Perissi Lab is dissecting the molecular mechanisms that control adaptive responses to metabolic stress and pro-inflammatory signals in adipocytes and immune cells.
A major area of interest is nuclear-mitochondrial communication. We recently identified G-Protein Suppressor 2 (GPS2) as a mediator of mitochondrial retrograde signaling and a required transcriptional cofactor for the expression of nuclear-encoded mitochondrial genes. Our data indicate that GPS2 regulated translocation from the mitochondria to the nucleus is critical for responding to acute mitochondrial stress and for sustaining mitochondrial biogenesis during adipocyte differentiation (Cardamone et al., Mol Cell, 2018).
We also focus is on non-proteolytic K63 ubiquitination as a key regulatory event for cellular adaptation. This stems from the identification of GPS2 as a specific inhibitor of the ubiquitin-conjugating enzyme Ubc13 (Lentucci et al., JBC, 2017). Investigating the role of the GPS2-Ubc13 module within different cellular compartments led us to describe an unexpected role for GPS2 as a suppressor of PI3K/AKT signaling downstream of the insulin receptor and as an inhibitor of TLR and TNFR pro-inflammatory signaling pathways (Cardamone et al., Molecular Cell, 2012; Cederquist et al., Molecular Metabolism, 2016; Lentucci et al., JBC, 2017). GPS2-mediated inhibition of Ubc13 activity is also important for chromatin remodeling and priming of target gene promoters in the nucleus (Cardamone et al., Cell Reports, 2014; Cardamone et al., Mol Cell, 2018).
Ongoing studies investigate the relevance and synergism among these functions in the context of obesity-associated inflammation/insulin resistance and breast cancer. Techniques routinely employed in the lab include basic molecular biology and biochemical approaches (site-specific mutagenesis, CRISPR editing, gene expression analyses, protein-protein interaction studies, in vitro enzymatic assays), next generation sequencing (ChIPseq, RNAseq, GROseq) and in vivo studies (mouse models of tissue-specific GPS2 knock-out).