Metallobiochemistry of Carbon
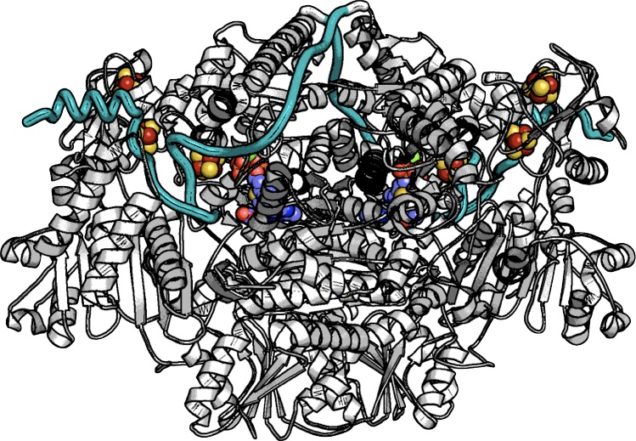 The most recent major effort of the Elliott Group is on the topic of the redox enzymes that are associated with CO2 transformations: in particular reversible enzymes that are capable of either producing the green-house gas in an oxidative fashion, or, reducing CO2 to either a fuel or another biochemical intermediary. One such class of enzyme is exemplified by the oxo-acid ferredoxin oxidoreductases (OFORs) which can be found in bacteria and archaea, either oxidizing oxo-acids in order to producing two reducing equivalents in the form of a coupled Ferredoxin (Fd) protein, or, OFORs are found in the reverse TCA cycle, in which they take up two electrons from an Fd and CO2, and produce molecules like pyruvate or oxo-glutarate. We are interested in how these reversible catalysts work, at the level of electron transfer events, redox potentials, and recognizing substrates and partner Fds. By bringing our unique experience with protein electrochemistry to bear upon OFORs and other enzymes, we hope to illustrate just how nature tunes the bias of an enzyme to favor CO2 reduction, over its production. Here we are fortunate to have another set of great collaborators, such as Prof. Catherine Drennan (HHMI/MIT), Prof. Stephen Ragsdale (UMich), Prof. Russ Hille (UC-Riverside), and Prof. Volker Müller (Frankfurt).
The most recent major effort of the Elliott Group is on the topic of the redox enzymes that are associated with CO2 transformations: in particular reversible enzymes that are capable of either producing the green-house gas in an oxidative fashion, or, reducing CO2 to either a fuel or another biochemical intermediary. One such class of enzyme is exemplified by the oxo-acid ferredoxin oxidoreductases (OFORs) which can be found in bacteria and archaea, either oxidizing oxo-acids in order to producing two reducing equivalents in the form of a coupled Ferredoxin (Fd) protein, or, OFORs are found in the reverse TCA cycle, in which they take up two electrons from an Fd and CO2, and produce molecules like pyruvate or oxo-glutarate. We are interested in how these reversible catalysts work, at the level of electron transfer events, redox potentials, and recognizing substrates and partner Fds. By bringing our unique experience with protein electrochemistry to bear upon OFORs and other enzymes, we hope to illustrate just how nature tunes the bias of an enzyme to favor CO2 reduction, over its production. Here we are fortunate to have another set of great collaborators, such as Prof. Catherine Drennan (HHMI/MIT), Prof. Stephen Ragsdale (UMich), Prof. Russ Hille (UC-Riverside), and Prof. Volker Müller (Frankfurt).
This work is supported by the Department of Energy, through grant DE-SC00112598 .
Representative Publications
1. Ceccaldi P, Schuchmann K, Müller V, Elliott SJ, “The hydrogen dependent CO2 reductase: the first completely CO tolerant FeFe-hydrogenase,” Energy and Environmental Science , 2017, 10, 503-508. [DOI]
2. Li B and Elliott SJ. “The catalytic bias of 2-oxoacid:ferredoxin oxidoreductase in CO2 evolution and reduction through a ferredoxin-mediated electrochemical assay,” Electrochimica Acta, 2016, 199, 349-356. [DOI]