Research
The research efforts of our group center largely on blood-tissue interfaces – the anatomical sites where blood and immune cells, and various blood-borne factors (both endogenous and exogenous) encounter and/or engage diverse tissues and organs as they circulate throughout the body. These interfaces can function as gates regulating tissue access; the cellular and molecular nature of these gates can vary widely and is often directly linked to organ-specific functions (e.g., the highly-restrictive blood-brain-barrier or the more permeable and heavily-trafficked sinusoidal endothelium of the liver and bone marrow). We aim to understand differences in the blood-tissue interface of distinct organs with the goal of exploiting unique molecular mechanisms to promote or inhibit the movement of cells into and out of specific tissues, or to guide the targeted delivery of therapeutic agents.
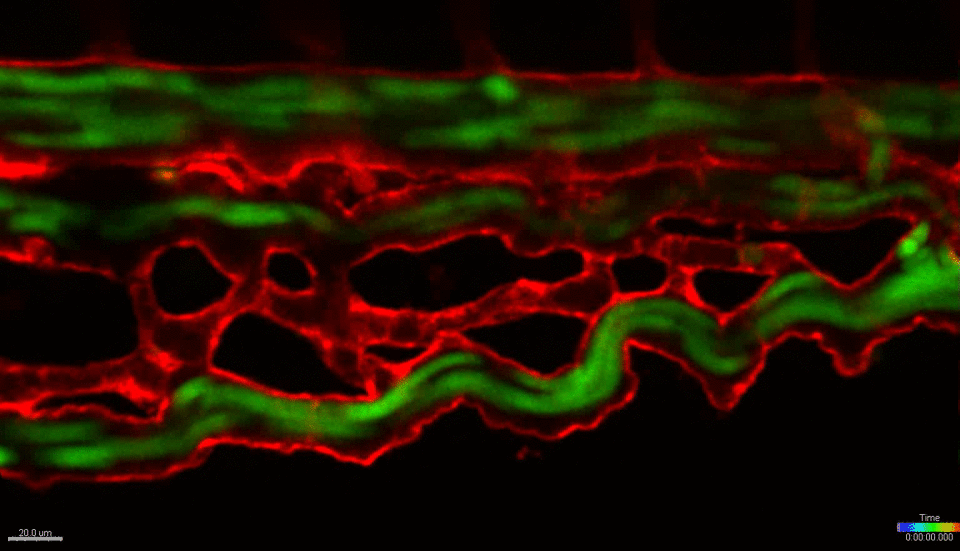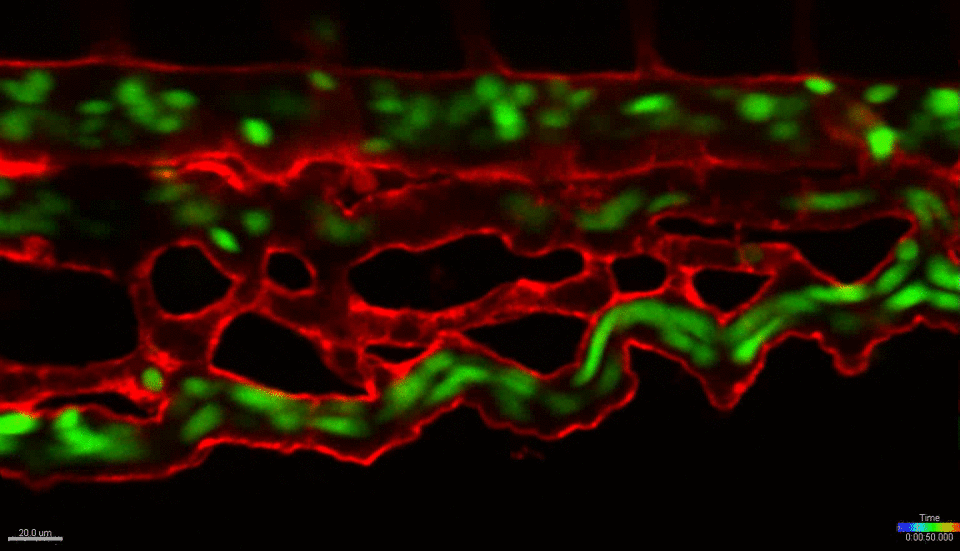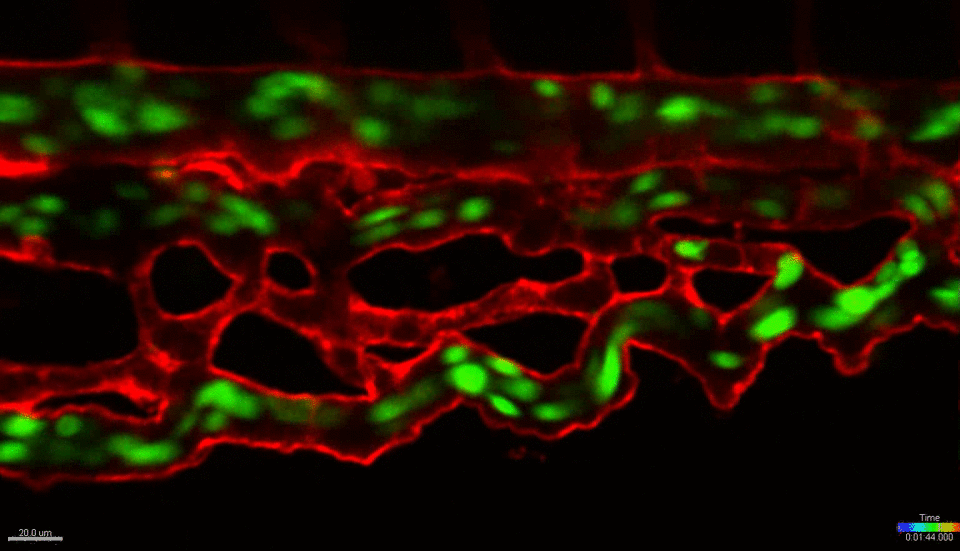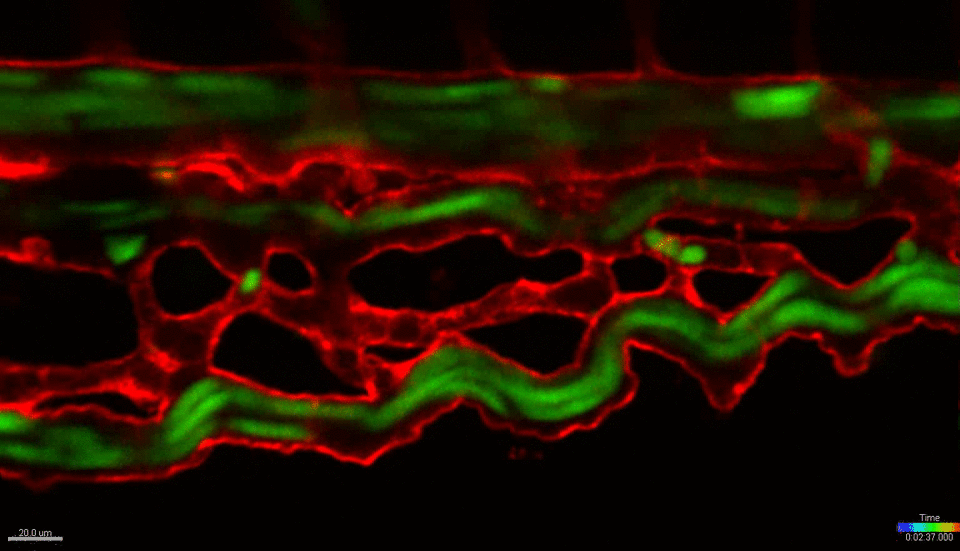
We use the zebrafish (Danio rerio) as a primary research model organism. The blood and vascular systems of the fish are highly similar to humans, while offering a unique experimental toolkit that includes the ability to perform high-throughput chemical and genetic screens, embryological manipulations and xenotransplantation (injecting cells from other species, such as mouse or human, into the fish). Their rapid and external development allows experiments to be performed across developmental time and across multiple scales, simultaneously – from whole organism, to individual organs, tissues, single cells and sub-cellular organelles. The transparent embryos allow direct visualization of cells in live animals at unprecedented resolution. Together, these attributes and the powerful tools of the system allow us to investigate fundamental biological mechanisms at a scale and resolution not possible in any mammalian model or in vitro/ex vivo system. We work closely with collaborators across the BU/BUMC campuses who use mouse models, in vitro culture systems and human patient tissues, with the goal of translating our basic finding in zebrafish towards the development of new therapies for treating human diseases.


Current projects:
1. Tissue- and cell-specific mechanisms of blood and immune cell migration
The goal of this project is to define the niche function of candidate molecules expressed specifically by vascular endothelial cells in the blood stem cell niche. By altering the associated pathways we aim to control the migration of distinct cell types (e.g. blood stem cells, macrophages or CAR T cells) as they move into and out of specific tissues, such as the bone marrow, brain and solid tumors.
2. Mechanisms of tissue-specific vascular endocytosis
The aim of this project is to define mechanisms of vascular endocytosis unique to specific tissues, including the blood stem cell niche and the blood-brain barrier, with the objective of modulating these mechanisms to control the migration of cells into and out of these tissues, or to deliver drug-based therapies (e.g., nanoparticles) in a targeted fashion.