Research
We have several projects in the lab that stem from interrelated lines of basic and translational research and use a variety of neuropathological, molecular, tract-tracing, high-resolution microscopy, imaging, and computational neural modeling and machine learning approaches. Below are a few examples, staring from basic to more translational research focused on disorders.
- Networks for flexible behavior and attention in the thalamus, amygdala, and cortex
- Comparative studies of brain networks in non-human primates and humans: a template for study of disorders
- Neuropathology and biomarkers of Autism Spectrum Disorders
- Mechanisms of disruption and biomarkers of Schizophrenia
- Mechanisms of disruption and biomarkers of Neurodegenerative Disorders
- Artificial Intelligence and Machine Learning for the study of Mental and Neurological Disorders
1. Networks for flexible behavior and attention in the thalamus, amygdala, and cortex
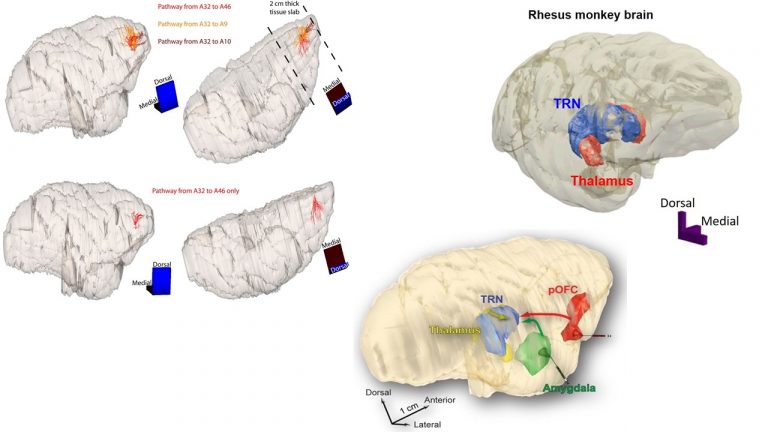
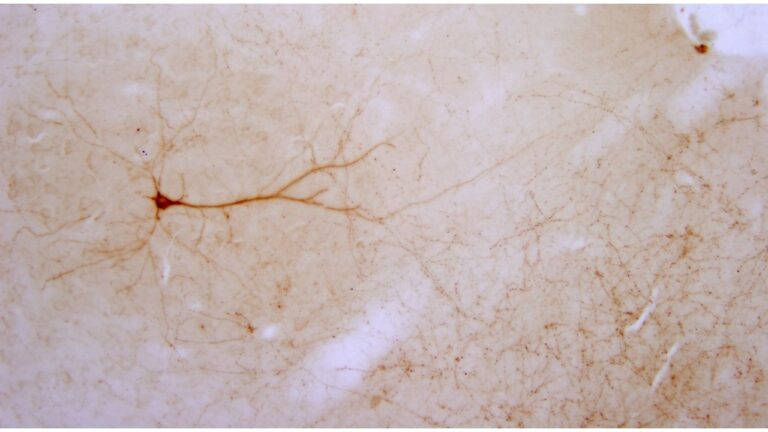
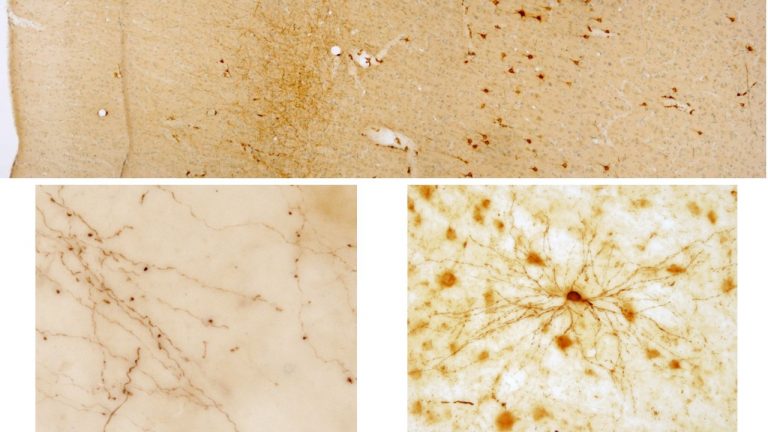
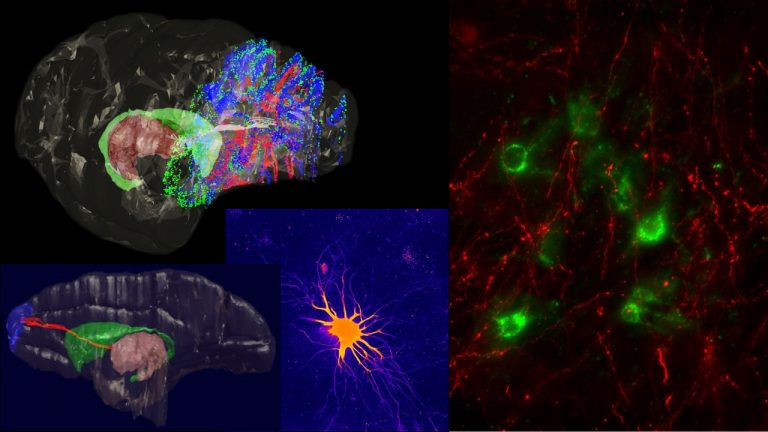
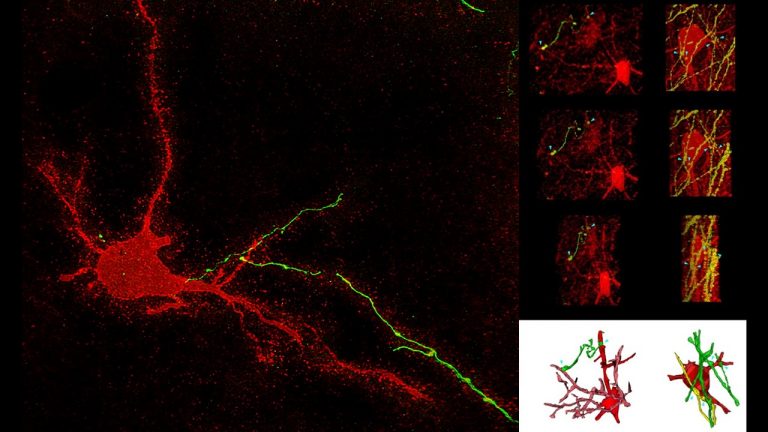
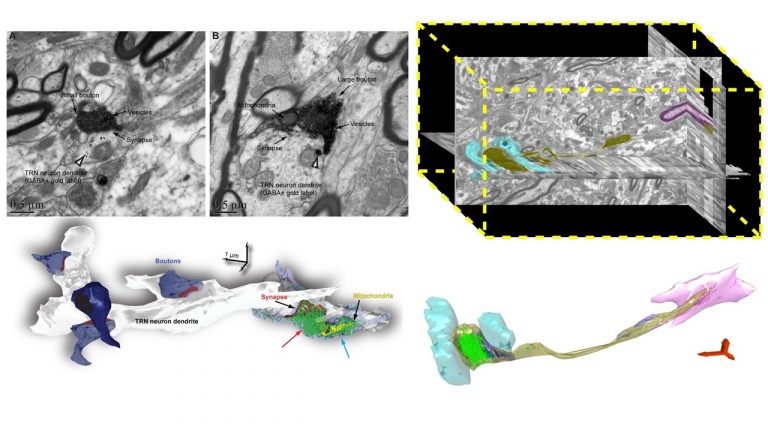
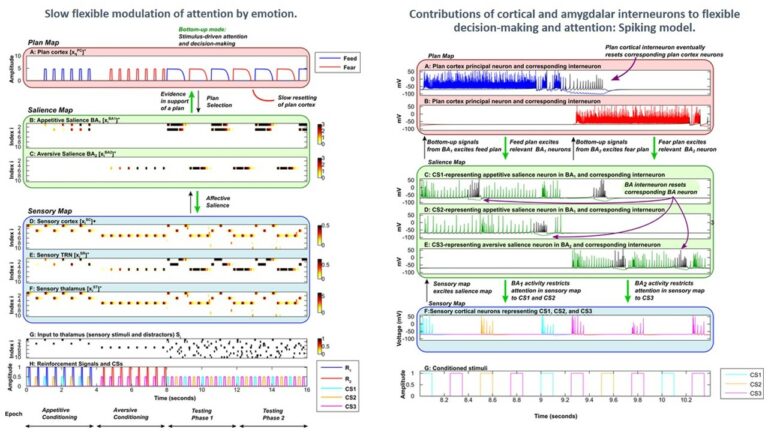
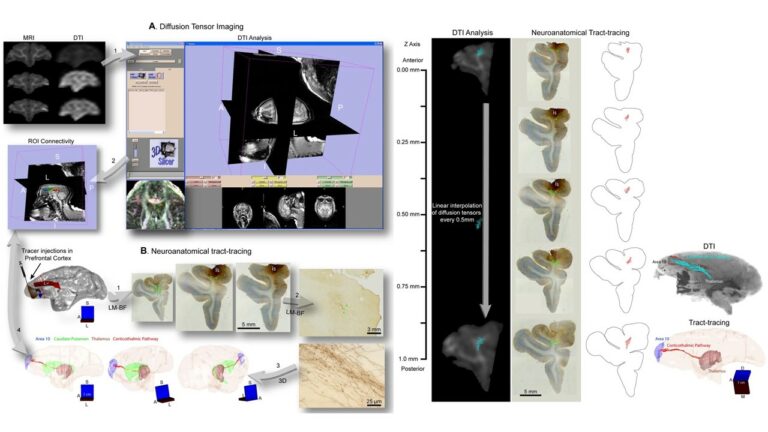
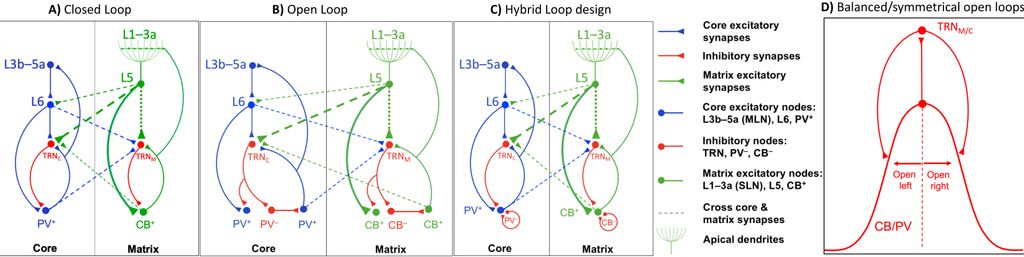
We study brain pathways and complex circuit interactions in networks linking prefrontal cortices, the amygdala, and the thalamus in non-human primates. These circuits underlie brain activity shifts that direct attention for flexible behavior. One emerging theme from this continuing work is the increased role of interactions between excitatory cortical and subcortical pathways with purely inhibitory systems in the thalamic reticular nucleus (TRN), and the intercalated masses in the amygdala (IM). Another major focus of our current research is to further study specialized excitatory-inhibitory interactions in the primate brain and investigate how cortical pathways interact with high-order thalamic nuclei for flexible behavior. We study distinct cell type and synaptic interactions in each node of these circuits, using tract-tracing, cutting-edge, high-resolution imaging and large-scale 3D-reconstruction analysis. Experimental approaches are complemented by computational modeling to simulate network function and dysfunction in psychiatric diseases.
2. Comparative studies of brain networks in non-human primates and humans: a template for study of disorders
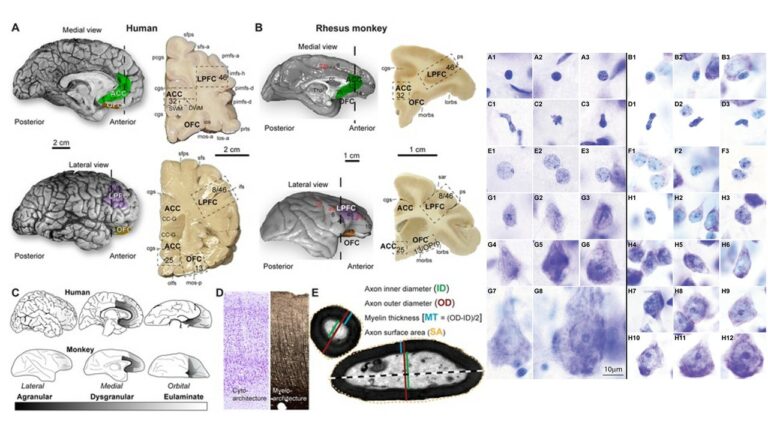
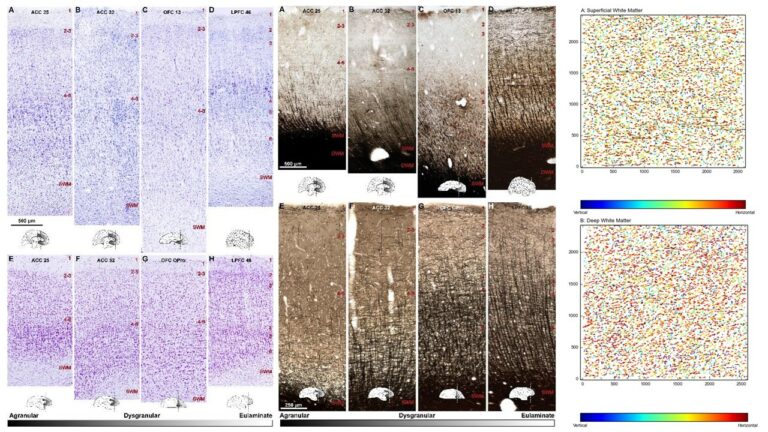
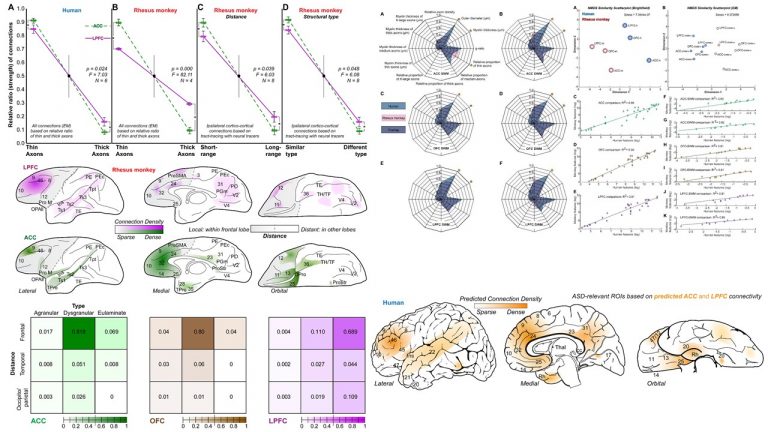
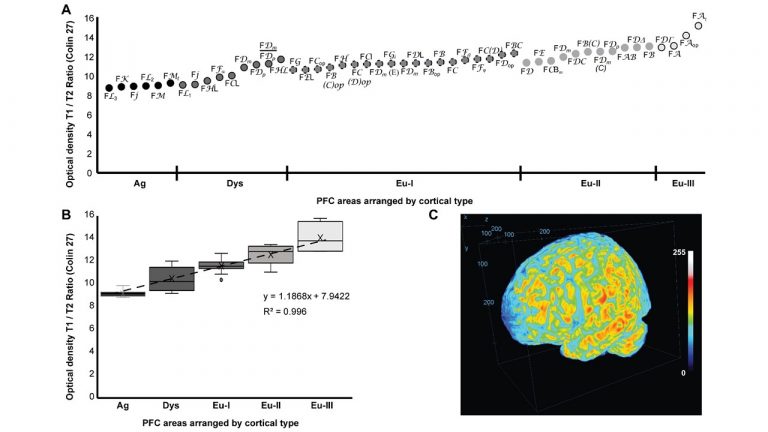
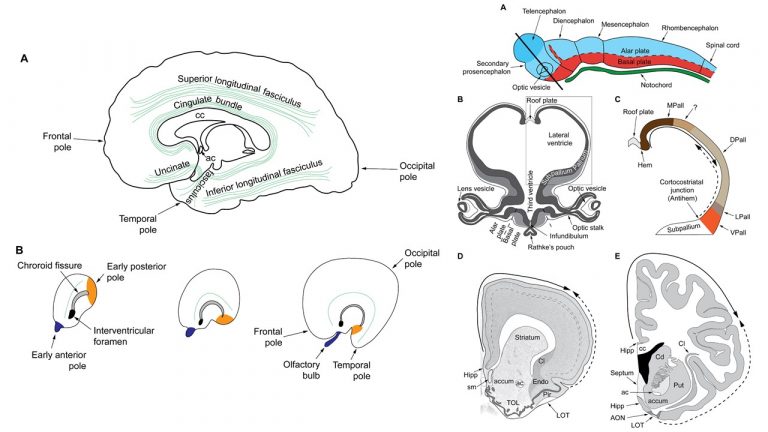
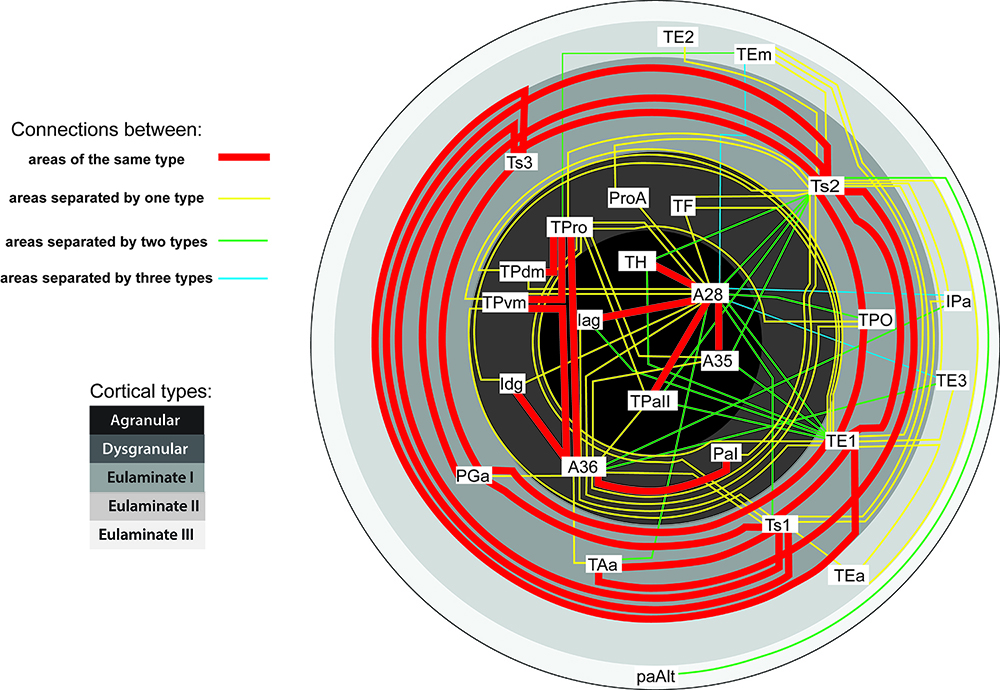
A major focus of our work revolves around an important unanswered question: Can the wealth of information on the structure and connections of the brain, derived from animal studies, be translated to understand neural communication in humans and disruption in brain diseases? Our goal is to use high-resolution approaches to identify similarities and key differences in cellular, synaptic, and molecular features of cortical and subcortical networks in humans and non-human primates. The aim is to develop reliable templates to translate high-resolution data on connections and circuit interactions from non-human primates and other mammals to humans, through side-by-side study of the structure and connections in these species at multiple scales. Our ongoing work is increasingly focusing on linking (epi)genetics with the anatomical and functional organization of corticocortical and thalamocortical pathways. The aim is to facilitate study of complex disorders through a novel framework that can be used to predict connections in humans, where invasive connectivity studies are precluded.
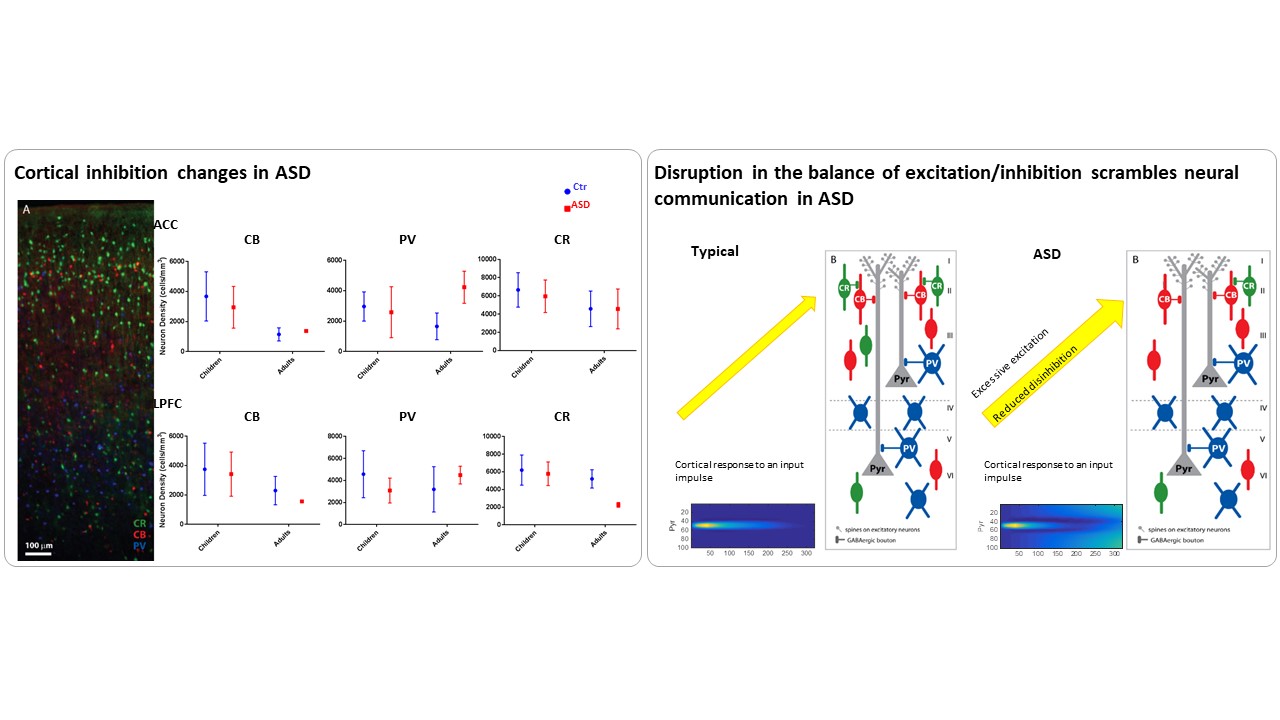
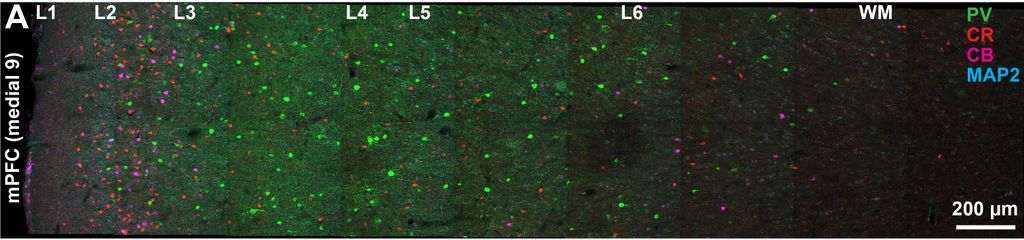
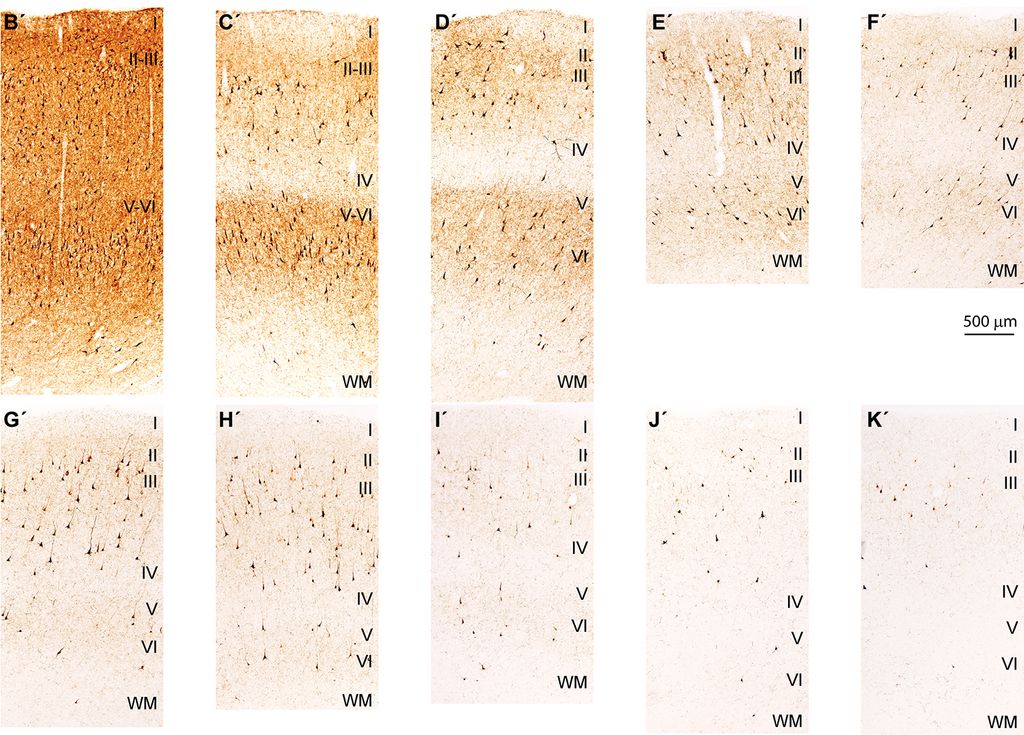
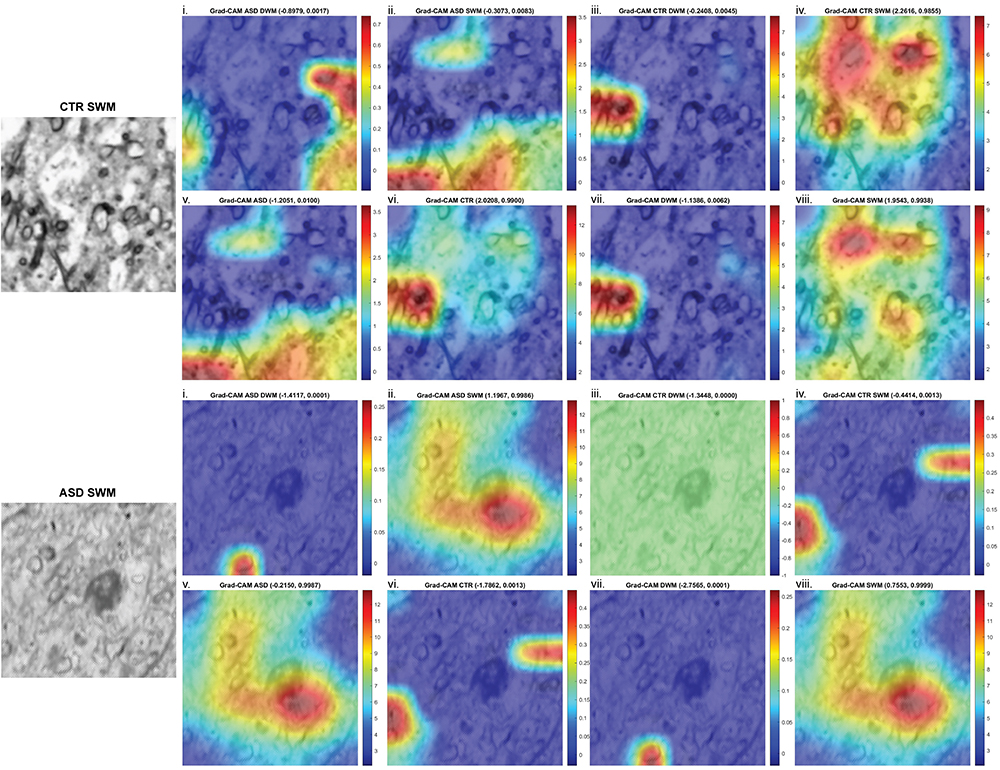
3. Neuropathology and biomarkers of Autism Spectrum Disorders
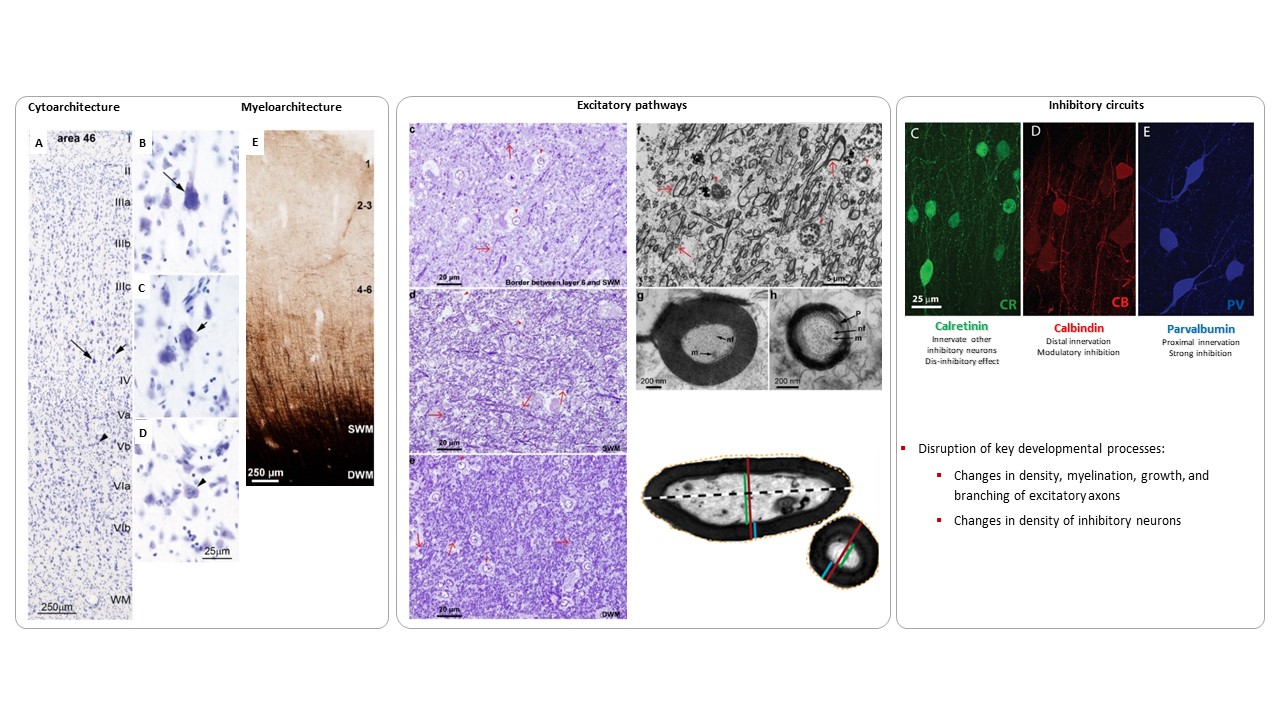
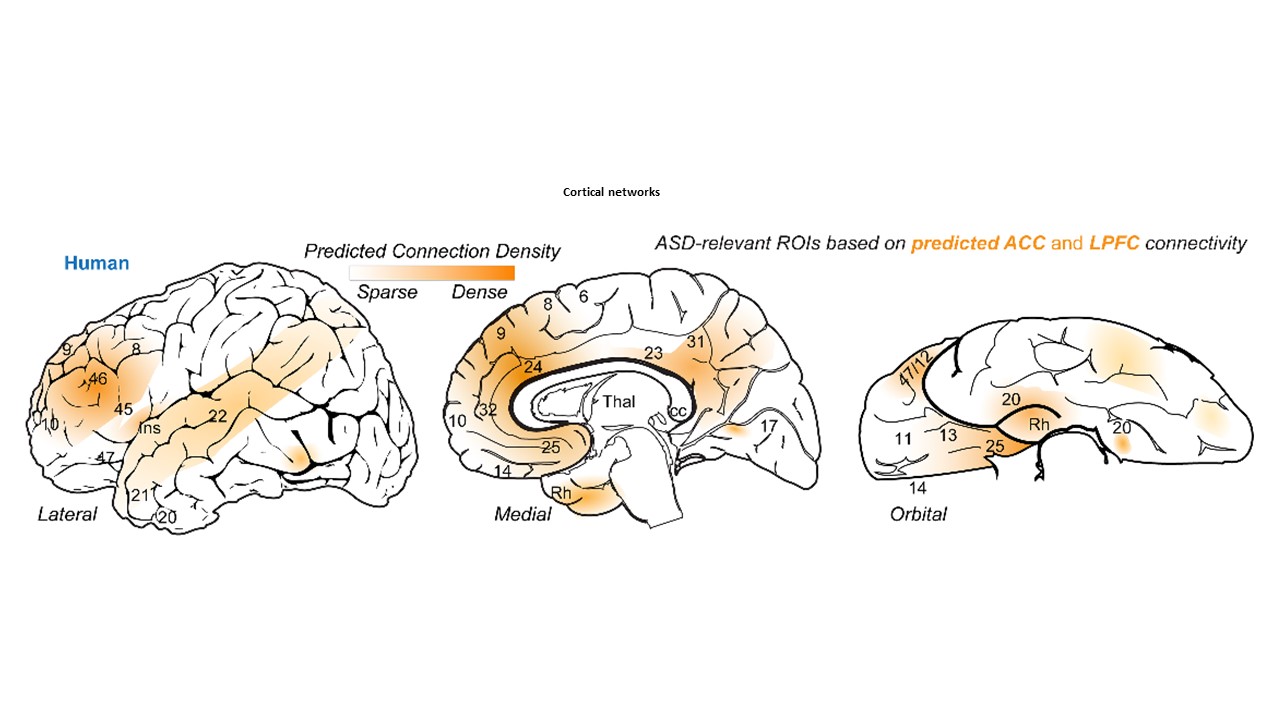
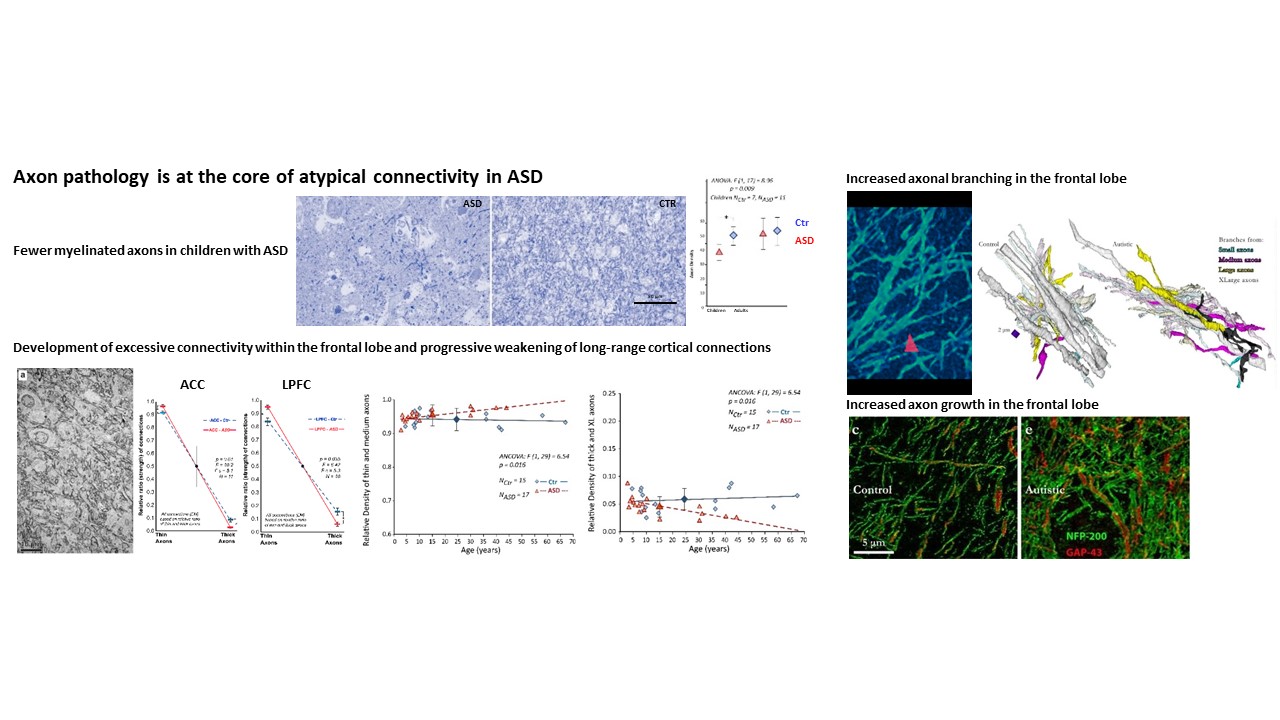
Using the template described above, we study the development of underlying pathology in functionally distinct feedforward, feedback, short-, or long-range brain pathways in individuals with autism spectrum disorders. For our neuropathological studies we use donated post-mortem brain tissue from adults and children. We model networks that have a role in directing attention, through thalamus and frontal cortex, to mediate social interactions and modulate emotional responses. We use circuit data and modeling to test hypotheses about mechanisms of disruption in neural communication at the level of cells, synapses and the larger system. We additionally link (epi)genetics with cellular processes and network disruption to elucidate the development of autism and inform potential interventions. We are also probing distinct disruptions in cortico-thalamic networks that are consistently affected in autism along with comorbid disorders, including depression, anxiety, schizophrenia, and epilepsy.
4. Mechanisms of disruption and biomarkers of Schizophrenia
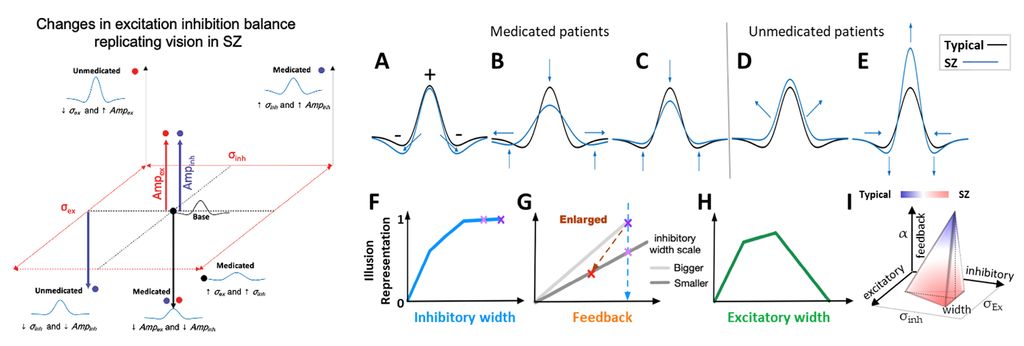
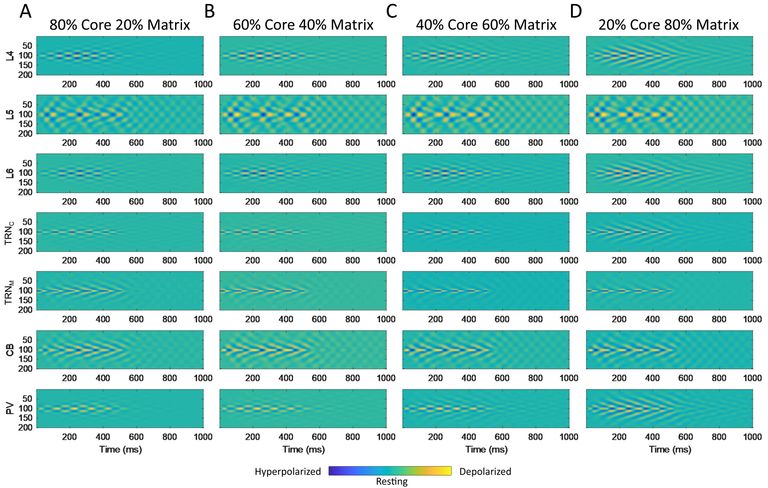
Within the same framework, and using similar approaches, we study the neural mechanisms underlying heterogeneous disruption of visual and sensory processing as well as as learning, memory, other executive functions, and sleep in schizophrenia (SZ). One perceptual disruption that we investigated is the altered sensitivity to visual contrast, which can be measured both in neurotypical observers and those with SZ. We used available published data that indicates overall higher and lower than typical visual contrast sensitivity in unmedicated and medicated patients with SZ, respectively, to develop a base neural model of the primate visual system. We used the model as a platform to correlate physiological and functional parameters, in particular those related to the neurobiological attributes of excitation and inhibition to replicate typical vs. SZ visual brain representations of contrast. We considered key physiological and neurobiological attributes, including the extent (spread) and strength of excitation, inhibition, and their ratios, which are directly associated with changes in the density, synaptic interactions, and expression of neurotransmitters and receptors of excitatory and inhibitory neurons. Through stepwise parametric alteration and testing of excitation / inhibition factors, and comparison of neural model representations of perception, we provided a superset of excitation/inhibition deviations that could replicate modified perception of visual contrast in medicated and unmedicated SZ patients. We then linked our heterogeneous superset of neurobiological possibilities with the general perceptual, behavioral, and neural traits of SZ. In this regard, we show that the altered percept of visual contrast in individuals with SZ is an aperture to broader system-level changes underlying neural processing in SZ. We are also using novel data on the organization and connectivity of circuits that link the thalamus and cortex to develop computational models that can simulate brain oscillatory activity and sleep spindles in humans. Our work has established a framework for the detailed study of the dynamics of variable types of sleep spindles and their disruption that can lead to deficits in sleep, memory, and attention.
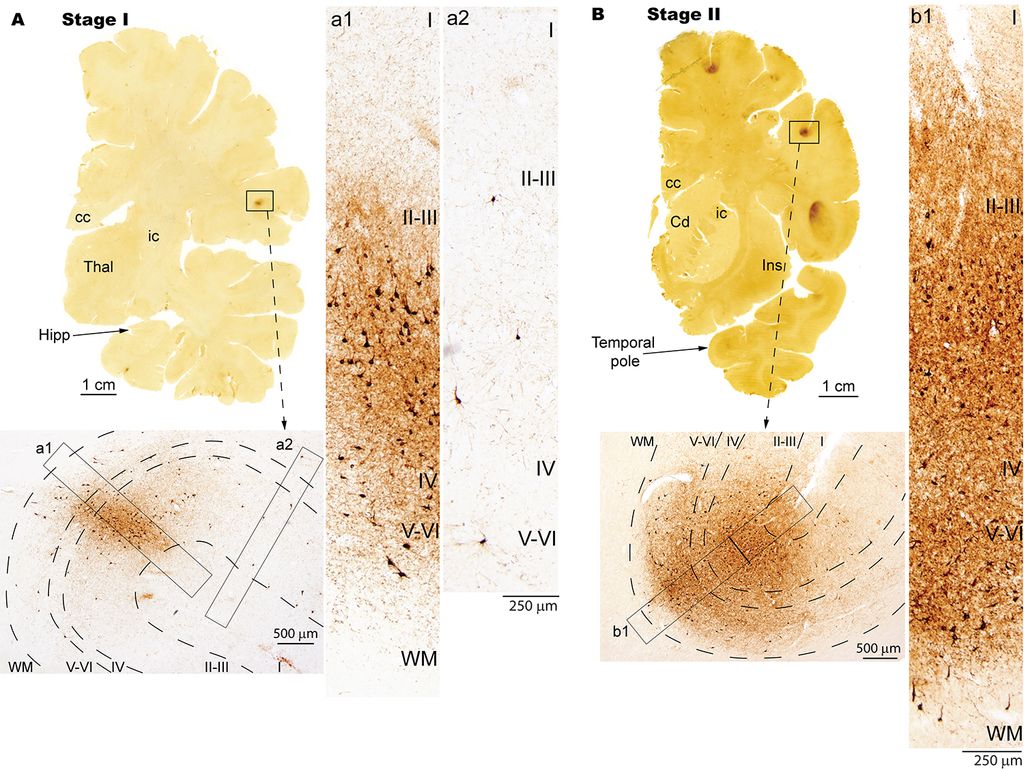
5. Mechanisms of disruption and biomarkers of Neurodegenerative Disorders
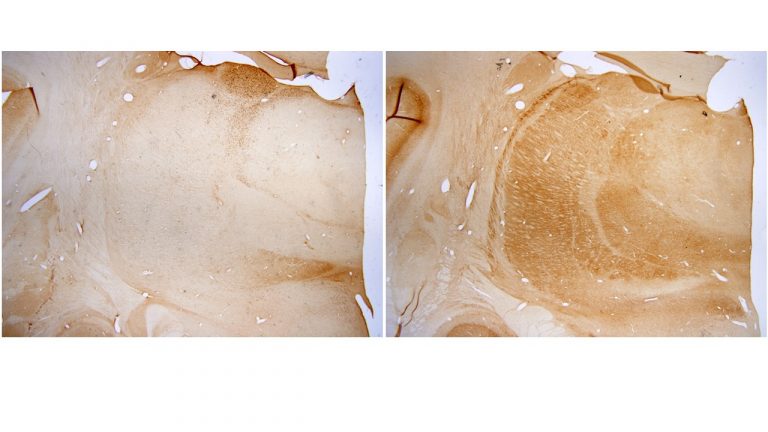
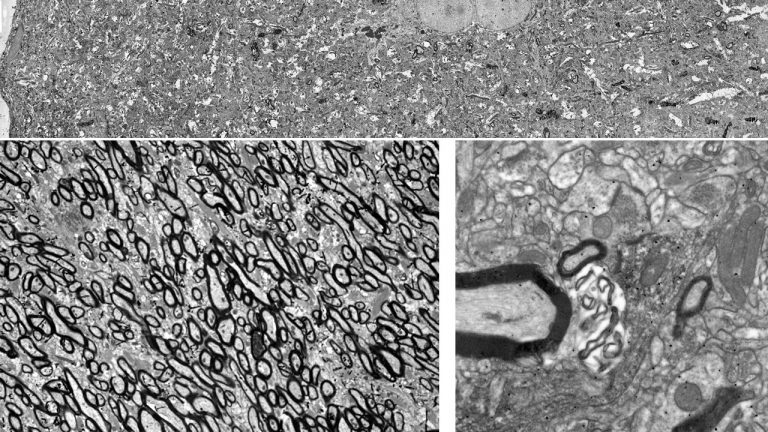
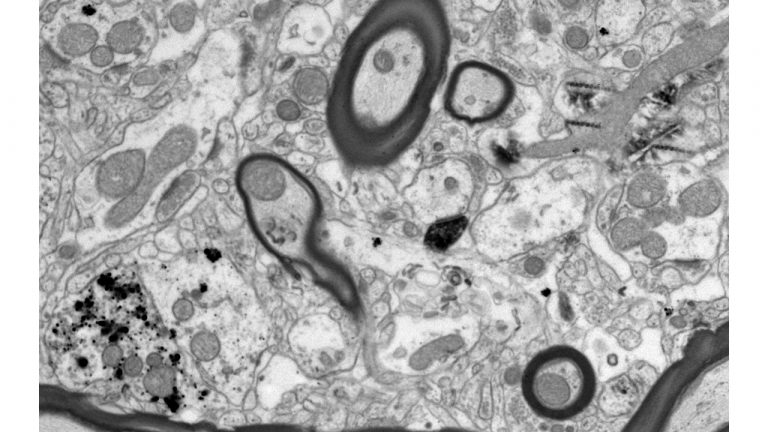
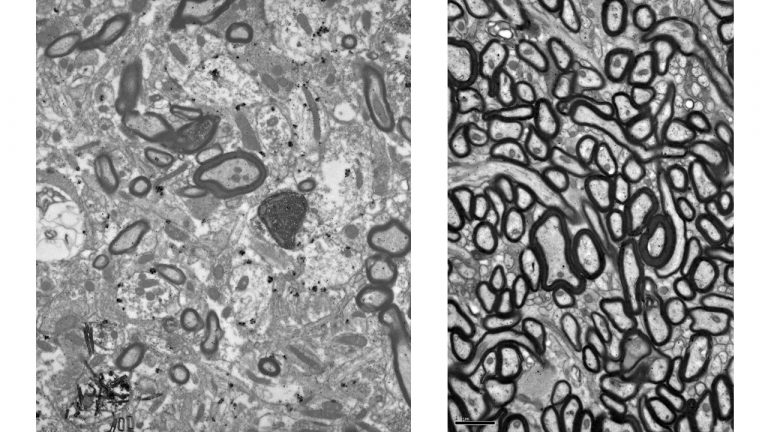
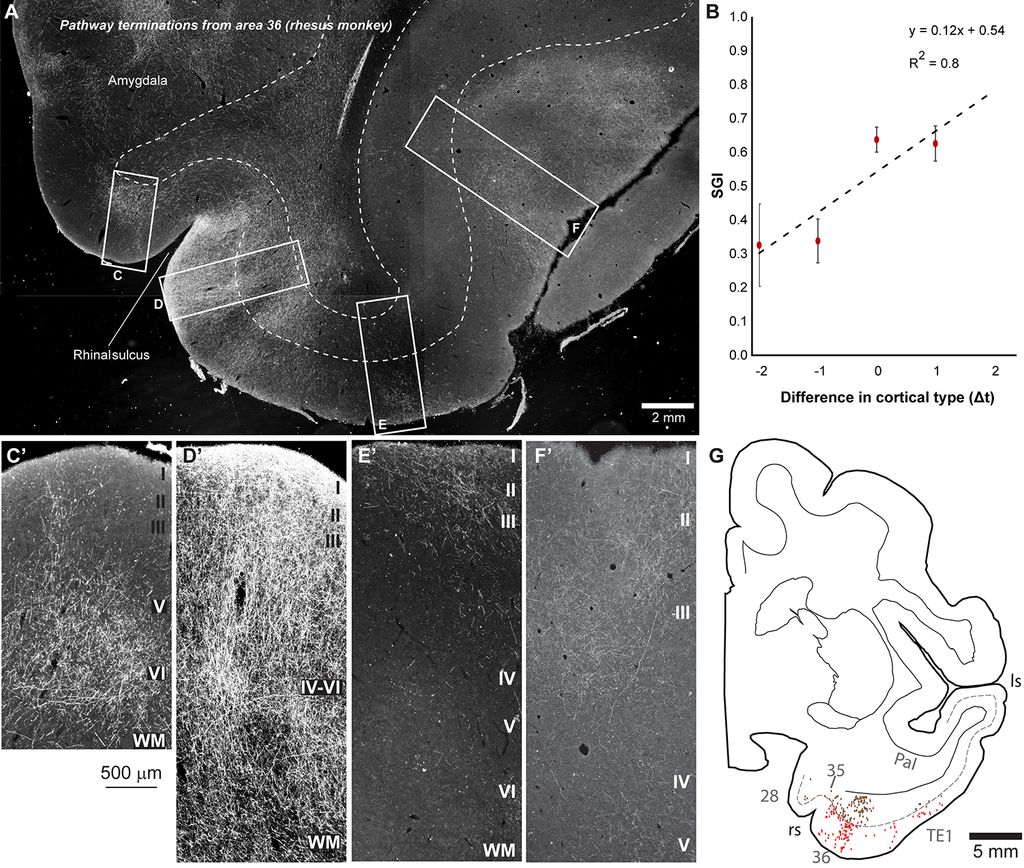
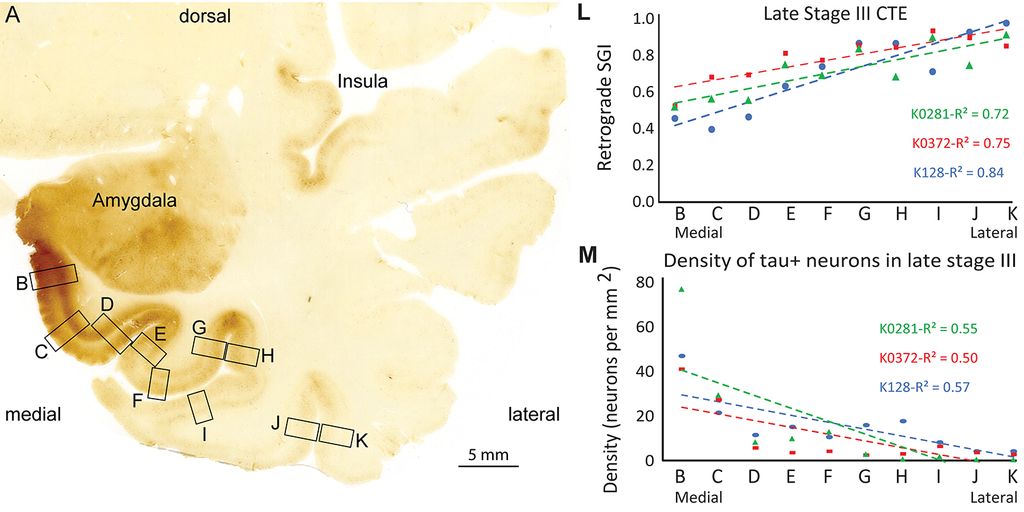
Using neuropathological and computational approaches we study the changes in brain networks after repetitive head trauma e.g., in Chronic Traumatic Encephalopathy (CTE), or in Alzheimer’s, and Parkinson’s diseases. In CTE, we found that spread of pathology in cortical neurons and axons followed connectivity rules seen in homologous cortical connections in non-human primates and can be predicted by a theoretical and computational model we developed. Our findings may help explain the inexorable spread of pathologic tau to widespread cortical areas accompanied by decline in emotional and cognitive processes in humans with repetitive head trauma. Our findings also highlight that tau pathology proceeds in different directions in CTE and Alzheimer’s disease.
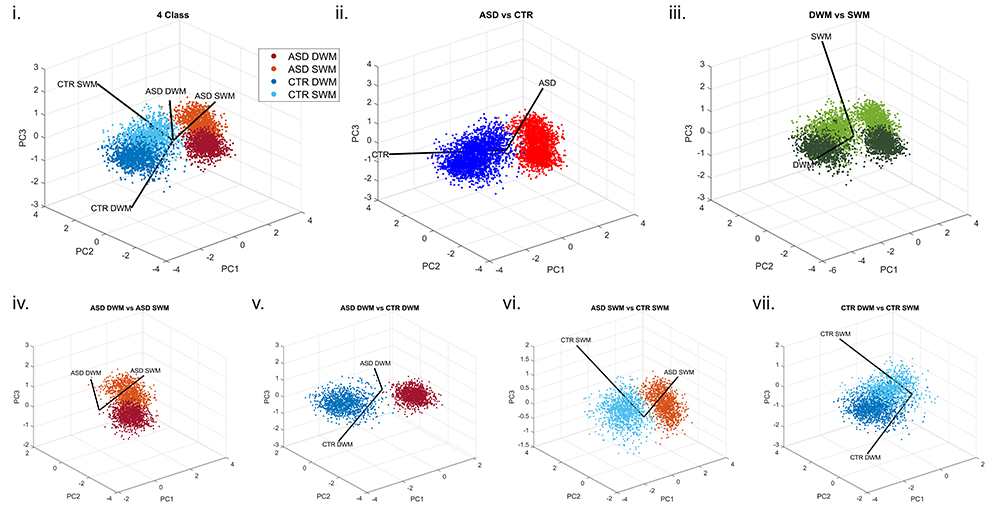
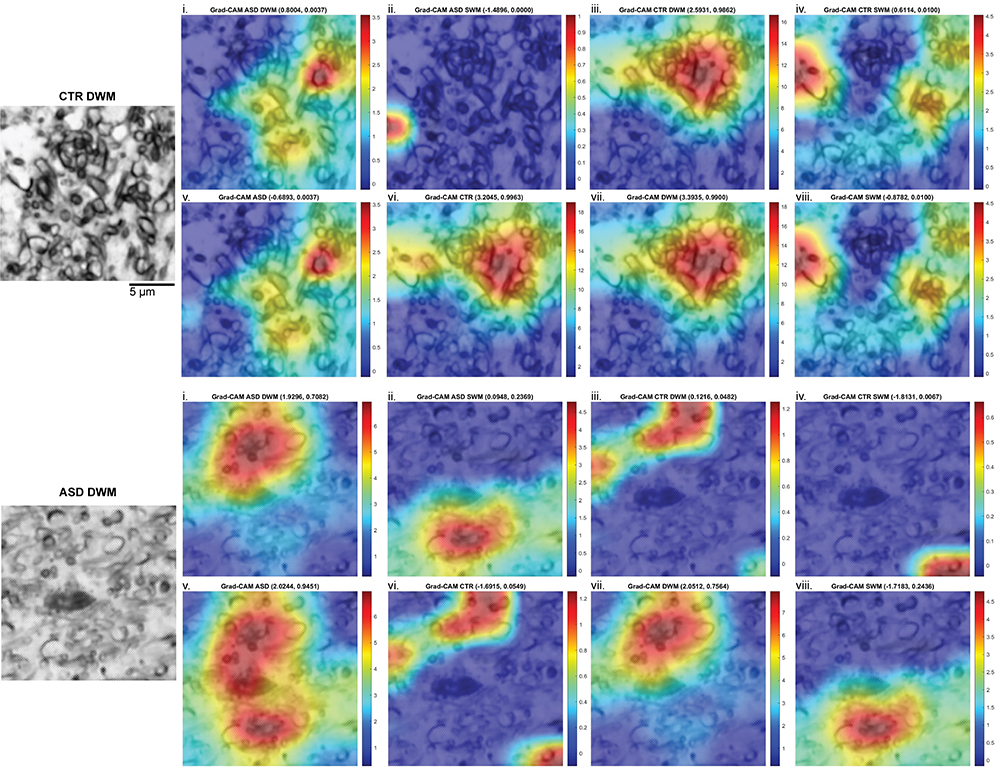
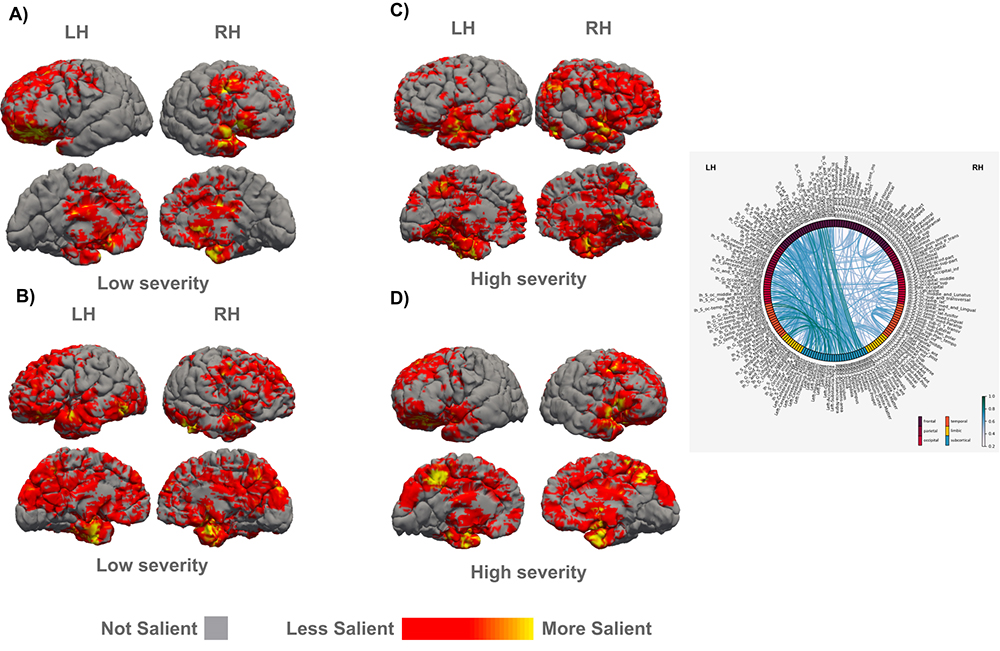
6. Artificial Intelligence and Machine Learning for the study of Mental and Neurological Disorders
We compile our detailed molecular, synaptic, cellular, circuit, and network data from neurotypical and neuropathological samples into large datasets and use machine learning to develop and optimize convolutional neural networks that can identify markers of pathology and disease subtypes. We use similar approaches to analyze structural and functional imaging (e.g., MRI, fMRI) datasets. Our goal is to develop and use explainable Artificial Intelligence (XAI), to then facilitate and guide development of diagnostics and targeted therapeutics. Ongoing projects involve autism, schizophrenia, Alzheimer’s and Parkinson’s disease.