bCCP Superfamily
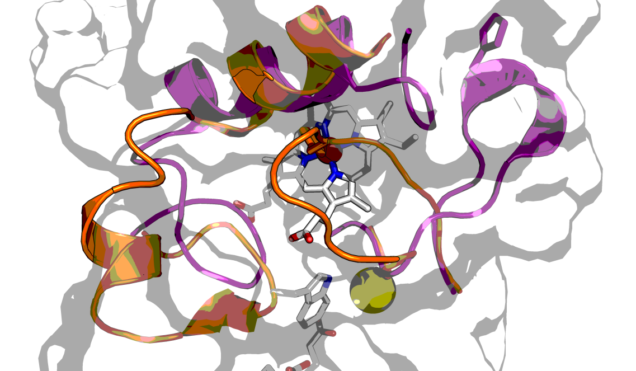 Diheme enzymes of the bacterial cytochrome c peroxidase (or bCCP) superfamily, span many types of microbial organisms. In each case reported thus far, two heme cofactors (which contain iron) are used jointly to attain two-electron chemistry, such as the reduction of hydrogen peroxide to water. We have worked over time to understand the structure:function relationships found within canonical bCCPs (that serve as true peroxidases in their native organism) and other enzymes that are similar at the level of primary amino acid sequence, but vary in function. For example, many bCCP enzymes are readily oxidized by molecular oxygen to a state where both iron atoms are at level of Fe(III), and the enzyme adopts a conformation where the active site iron is ligated by a histidine residue. The active conformation of the enzyme is then achieved by reducing the “other” heme, which is associated withe electron transfer, and not substrate binding. Alternatively, other bCCP family members don’t have this trait, and instead are always in a conformation that allows for catalysis to proceed. We use a combination of biochemistry, structural biology, spectroscopy and electrochemistry to understand the nature of this activation trait, which call “reductive activation”. Similarly, these enzymes have proved a fantastic model system for understanding how Nature tunes and connects, the chemistry of not one, but two heme cofactors. We are fortunate to have many excellent collaborators on this front, such as the group of Prof. Catherine Drennan (MIT/HHMI), Prof. Michael Hendrich (CMU), and Prof. Nicolai Lehnert (UMich). This work is supported by the National Institutes of Health (NIGMS) through grant R01-GM110390.
Diheme enzymes of the bacterial cytochrome c peroxidase (or bCCP) superfamily, span many types of microbial organisms. In each case reported thus far, two heme cofactors (which contain iron) are used jointly to attain two-electron chemistry, such as the reduction of hydrogen peroxide to water. We have worked over time to understand the structure:function relationships found within canonical bCCPs (that serve as true peroxidases in their native organism) and other enzymes that are similar at the level of primary amino acid sequence, but vary in function. For example, many bCCP enzymes are readily oxidized by molecular oxygen to a state where both iron atoms are at level of Fe(III), and the enzyme adopts a conformation where the active site iron is ligated by a histidine residue. The active conformation of the enzyme is then achieved by reducing the “other” heme, which is associated withe electron transfer, and not substrate binding. Alternatively, other bCCP family members don’t have this trait, and instead are always in a conformation that allows for catalysis to proceed. We use a combination of biochemistry, structural biology, spectroscopy and electrochemistry to understand the nature of this activation trait, which call “reductive activation”. Similarly, these enzymes have proved a fantastic model system for understanding how Nature tunes and connects, the chemistry of not one, but two heme cofactors. We are fortunate to have many excellent collaborators on this front, such as the group of Prof. Catherine Drennan (MIT/HHMI), Prof. Michael Hendrich (CMU), and Prof. Nicolai Lehnert (UMich). This work is supported by the National Institutes of Health (NIGMS) through grant R01-GM110390.
Representative Publications
1. Frato KE, Walsh KW, Elliott SJ. “Functionally distinct bacterial cytochrome c peroxidases proceed through a common (electro)catalytic intermediate,” Biochemistry, 2015, 55(1),125-132. [DOI]
2. Levin BD, Walsh KA, Sullivan KK, Bren KL, Elliott SJ, “Methionine Ligand Lability of Homologous Monoheme Cytochromes c”, Inorganic Chemistry, 2015, 54 (1):38-46. [DOI]
3. Ellis KE, Frato KE, Elliott SJ. “Impact of Quarternary Structure upon Bacterial Cytochrome c Peroxidases: does homodimerization matter?” Biochemistry, 2012, 51(50): 10008-10016. [PDF]