Active Research Projects
Our research participants are partners in our mission to advance new rehabilitation interventions and technologies. If you or someone you know is interested in being a research participant, let us know here!
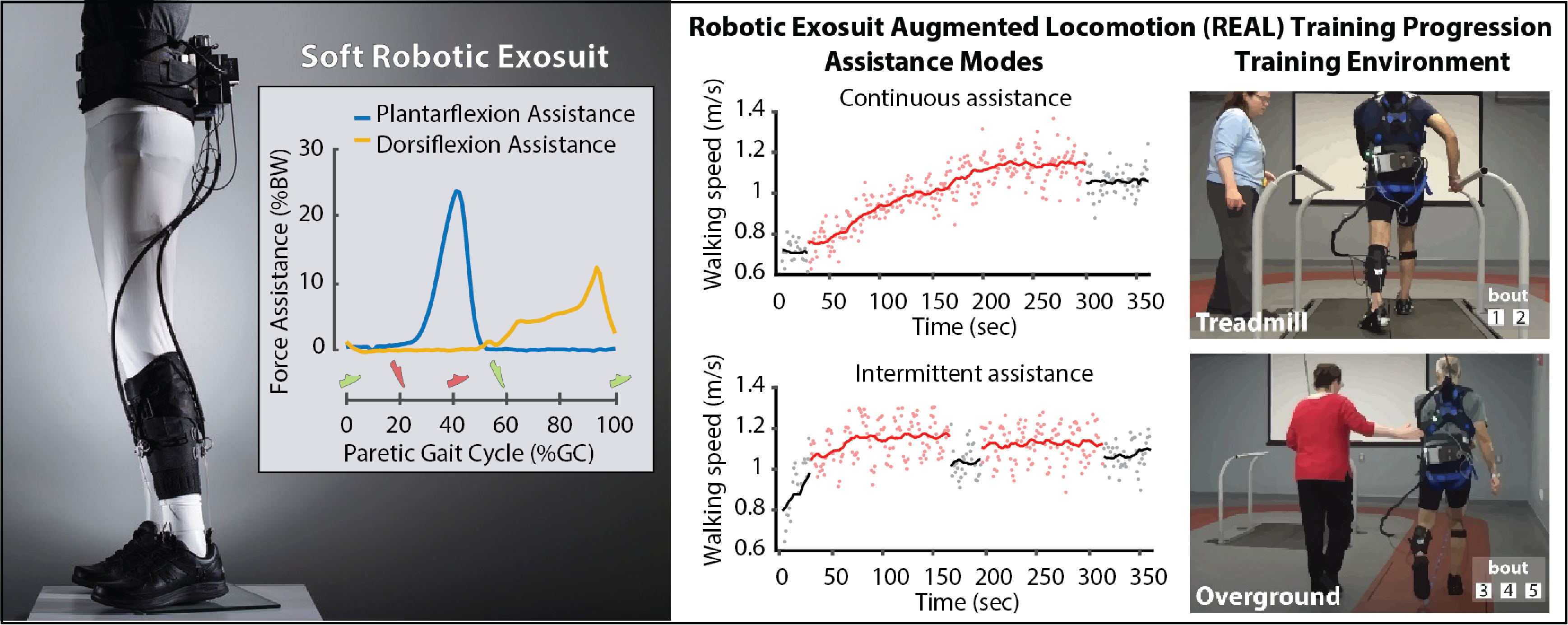
Robotic Exosuit Augmented Locomotion (REAL) gait training
Learn more here.
Slow walking speeds and impaired walking patterns are complex problems that persist after a stroke. Soft robotic exosuits have been shown to be capable of improving post-stroke walking patterns and walking speed when they are worn and powered on. We believe that these immediate gait benefits can be leveraged during high-intensity gait rehabilitation to facilitate durable, long-standing therapeutic improvements in both the speed and quality of post-stroke walking. To this end, we are developing and evaluating a standardized Robotic Exosuit Augmented Locomotion (REAL) gait training program centered on our team’s understanding of human-exosuit interaction and contemporary motor learning concepts.
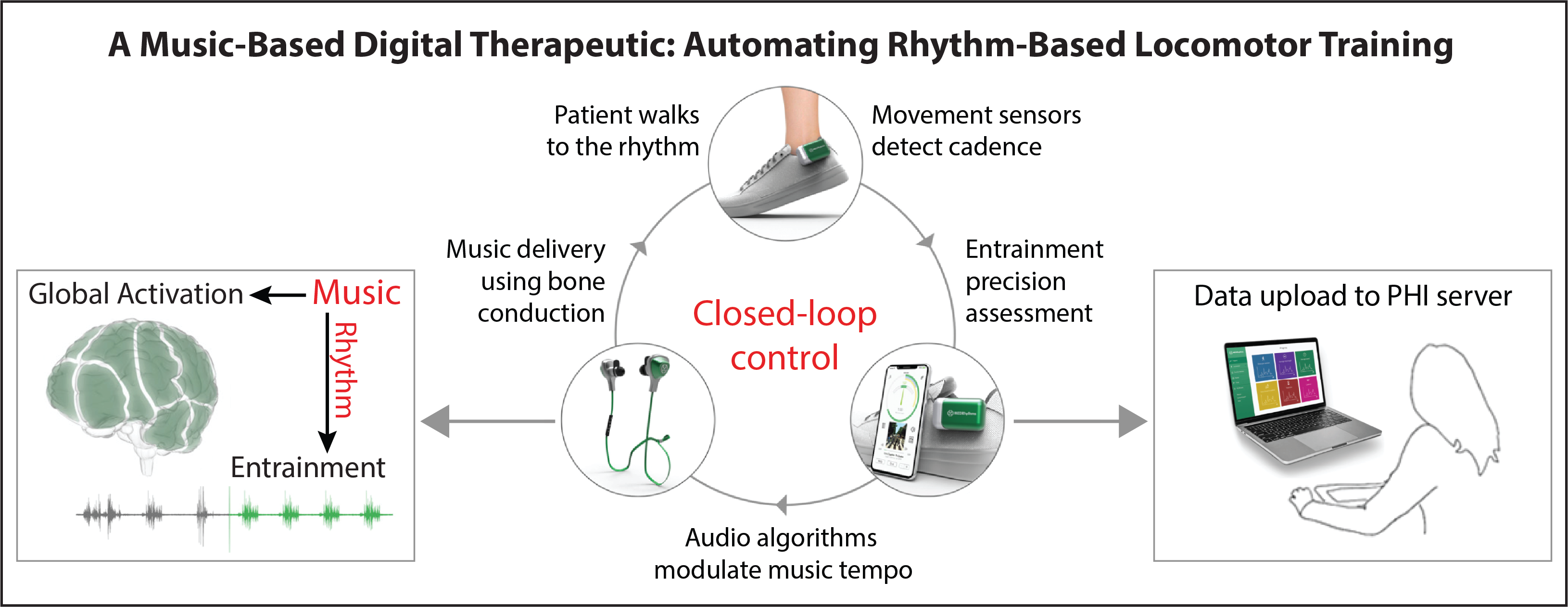
Walking to the beat of recovery: A music-based digital therapeutic
Learn more here.
We are studying a music-based digital therapeutic platform designed to leverage wearable movement sensor data to personalize a rhythm-based gait retraining program for patients with neuromotor impairments. When listening to music while walking, humans naturally change their walking rhythm to match the beat. The music-based digital therapeutic that we are studying can systematically alter the beat of popular songs (while preserving high sound quality) to help users change their walking rhythm, and ultimately their speed. If the user struggles to match the beat, automatic rhythm-assist algorithms kick in that fine-tune the musical tempo to better match the user’s abilities. Early findings from our laboratory have shown that this music-based digital therapeutic can automate a progressive and individualized rhythm-based walking training program that increases walking speed, reduces walking asymmetry, and decreases the energy cost of walking after stroke.
reNeu: A soft, hybrid robotic exosuit neuroprosthesis
Learn more here.
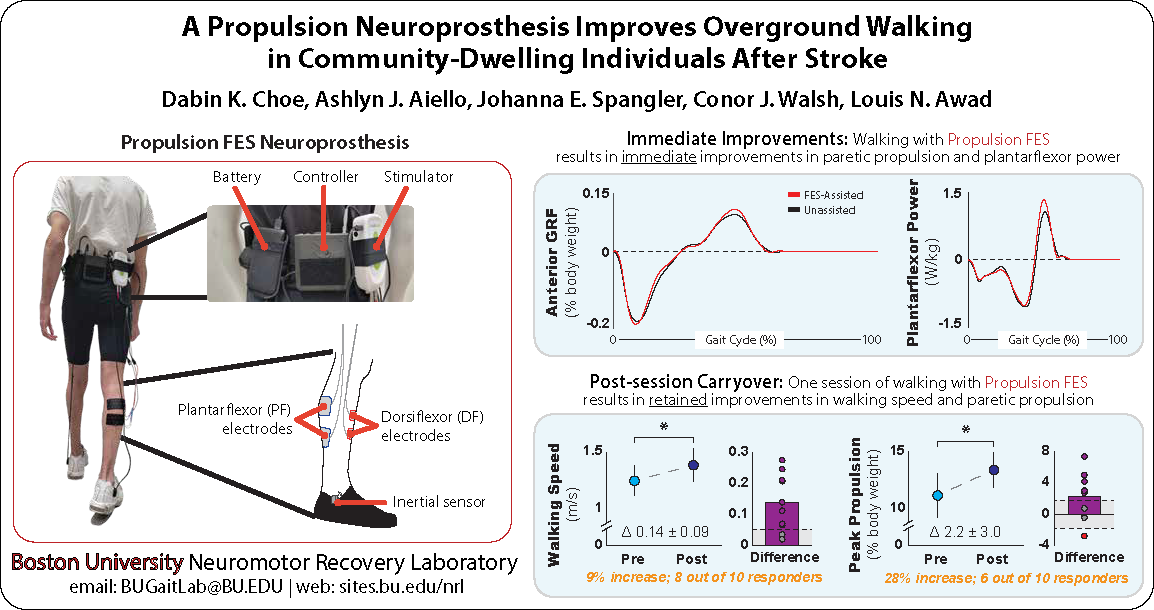
After stroke, muscles retain their ability to generate force, but the neuromuscular pathways that control brain-to-muscle communication are disrupted, causing many people with stroke to be unable to effectively access these pathways and control their muscles. To make use of residual muscle capacity and improve effective muscle control, we are developing a multi-channel functional electrical stimulation (FES) neuroprosthesis that can activate affected muscles and improve walking ability. The neuroprosthesis combines low-energy electrical pulses with sensor technology and adaptive gait algorithms to provide individualized lower extremity assistance during walking after stroke. We are also working to integrate this FES technology with soft robotic exosuits to create new hybrid assistance and rehabilitation systems for gait rehabilitation.
Digital movement phenotypes for precision rehabilitation
Learn more here.
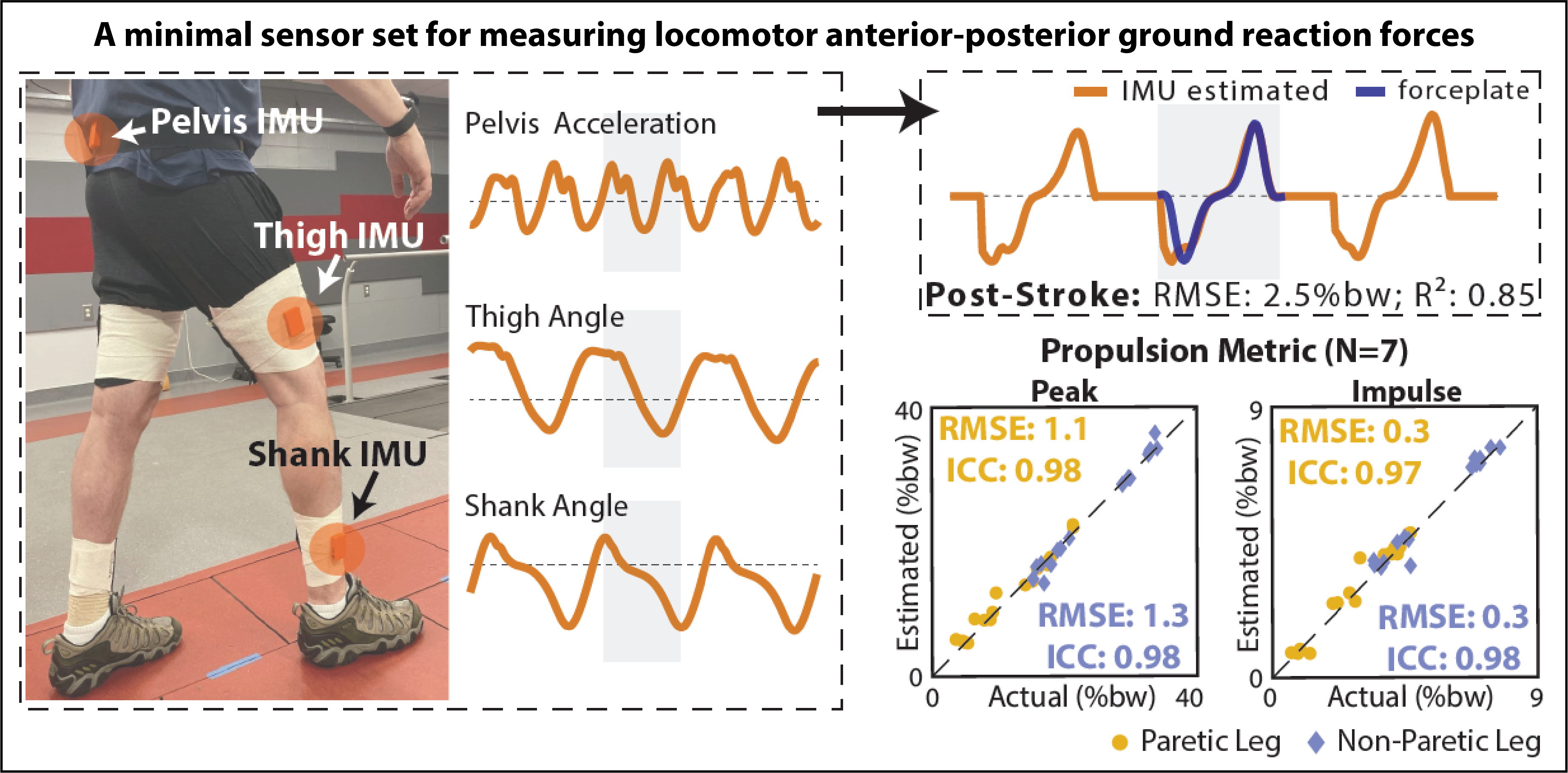
Among people with neurological injuries and diseases, different impairment patterns converge on a characteristically slow, asymmetric, and unstable gait. We seek to advance principles of precision medicine to the field of gait rehabilitation. This requires not only the development of targeted gait interventions, but also the advance of novel diagnostic methods that can efficiently match individual patients to those interventions. Before precision medicine is attainable in the field of gait rehabilitation, clinically-accessible classification methods that reflect patients’ underlying gait impairments are required. Indeed, the most common classification approach used post-stroke is based on walking speed, which is not indicative of underlying gait impairments. Inertial Measurement Units (IMUs) offer a promising solution for this measurement gap. IMUs can collect high-fidelity movement data continuously in the background of everyday walking activities and are already widely utilized to track step activity in both clinical practice and research. For this project, we are developing novel movement estimation algorithms using minimal IMU sets. Our goal is the advance of IMU-based classification of walking impairment based on both walking speed and gait quality. Because IMUs are inexpensive and can be used easily across both clinical and free-living settings, IMU-based locomotor phenotyping is highly scalable and has the potential to address the measurement gaps that have hindered progress in the field of gait rehabilitation.
BACPAC: Wearable robotic apparel for alleviating low back pain
Learn more here.
Over 80% of people experience low back pain in their lifetime, with 20% suffering recurrent episodes and progression to chronic pain. Pain, maladaptive motor control strategies, muscle spasm, declining capacity for activities of daily living, and development of fear-avoidance-beliefs contribute to failed rehabilitation and a vicious cycle of impairment. Unfortunately, the effectiveness of rehabilitative treatment can be limited. New robotic apparel technology (exosuit) can encourage safe movement strategies, reduce muscle exertion, and improve movement capacity through well-timed assistive forces to the trunk and hips during bent postures and lifting tasks. We believe this technology will enhance rehabilitation after back injuries and interrupt the progression to chronic pain and disability, by improving movement tolerance, reducing pain and fear with movement, and expediting return to prior level of function.
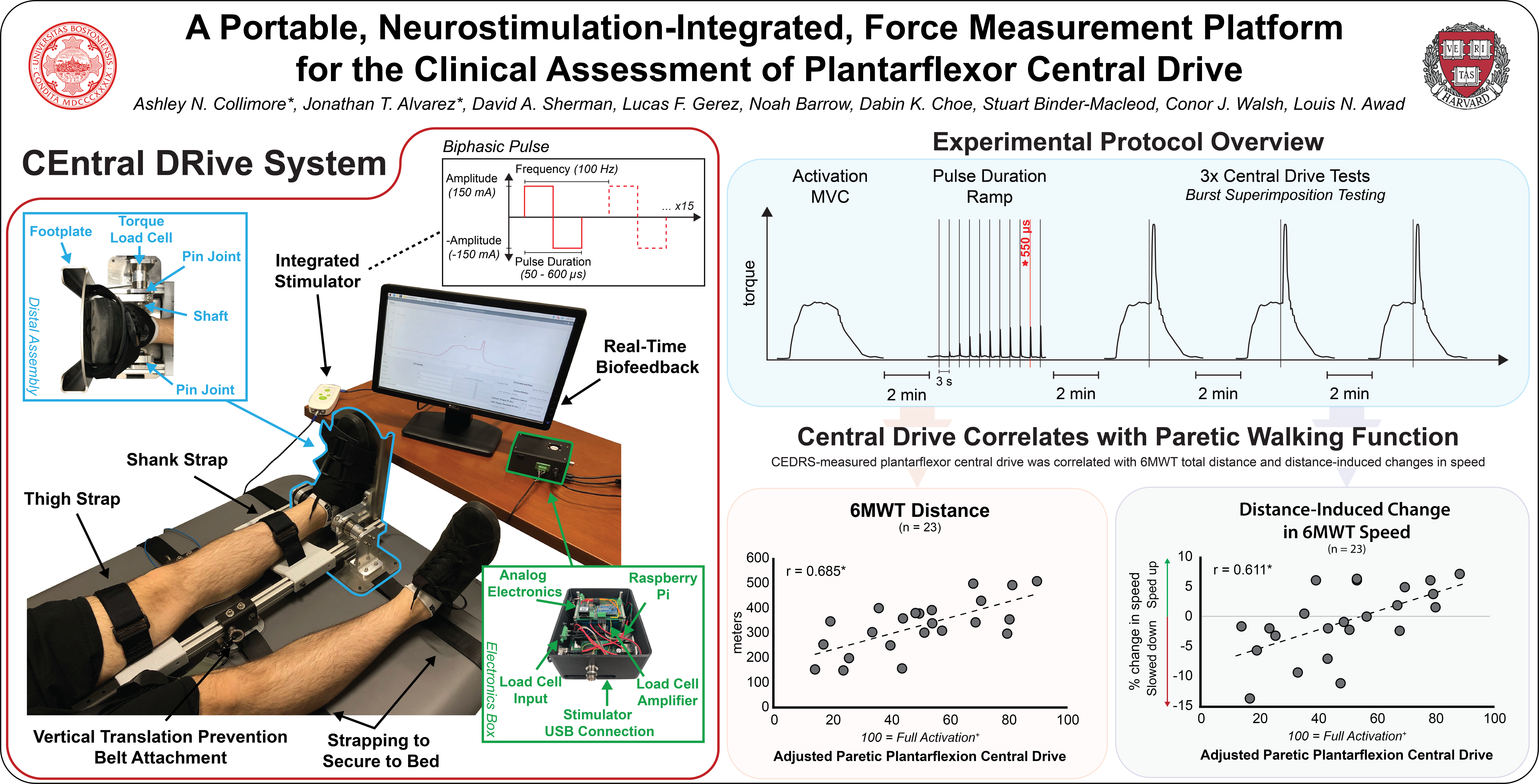
Neuromotor control after stroke: Understanding neuromechanical contributors to propulsion deficits
The plantarflexor muscles are the primary generators of propulsive force during walking. Post-stroke plantarflexor weakness may be the result of a reduced strength capacity (e.g., reduced physiological cross-sectional area due to muscle atrophy), reduced central neural drive, or a combination of these deficits. For individual patients, assessing each of these potential deficits is necessary to inform clinical decisions. A promising diagnostic approach to elucidate the extent and mechanisms underlying post-stroke plantarflexor muscle weakness combines dynamometry with supramaximal electrostimulation. Indeed, the maximum voluntary plantarflexor force that many community-dwelling individuals post-stroke are able to generate is only a fraction of their plantarflexor force-generating capacity. Our team has shown that the magnitude of this latent capacity (i.e., the central drive deficit) is a key explanatory factor of post-stroke propulsion impairments. Unfortunately, the diagnostic systems currently used for neuromuscular function testing require substantial time to set up and execute, as well as costly and large equipment not widely available in clinical settings. Together, these factors motivate the development of novel point-of-care plantarflexor force measurement systems.
EXO-WALK: Exosuit-Assisted Walking Automaticity and Locomotion Kinetics
Learn more here.
Stroke-induced brain injury to the neural pathways responsible for automatic walking also results in visible gait compensations—i.e., an asymmetrical, slow, and energetically effortful gait. Our team has developed a soft, wearable robotic exosuit that provides external mechanical assistance to the paretic limb, and we have shown walking with the exosuit reduces gait asymmetry, increases walking speed, and reduces walking effort. Moreover, users with post-stroke hemiparesis have subjectively reported improvements in walking automaticity during exosuit-assisted walking. In this study, we are seeking to use portable functional neuroimaging (functional near-infrared spectroscopy–fNIRS) to directly quantify the effects of the soft robotic exosuit on walking automaticity during overground walking, with testing both in our research laboratory in and in real-world settings.