Projects
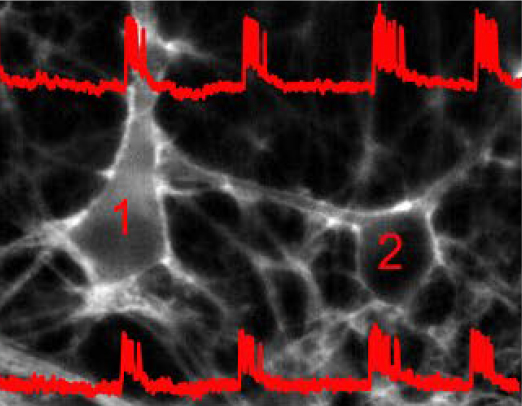
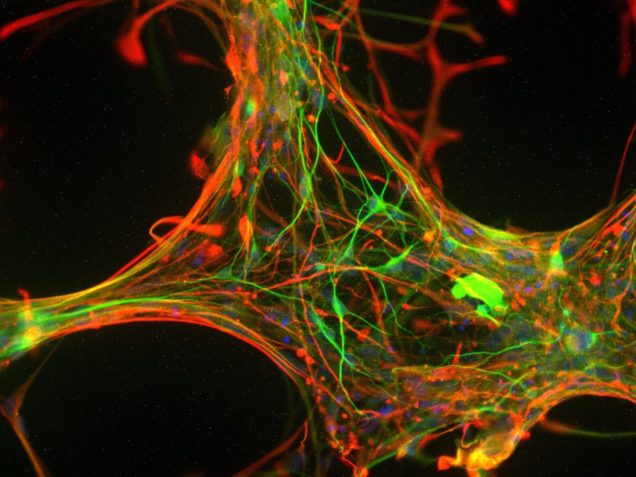
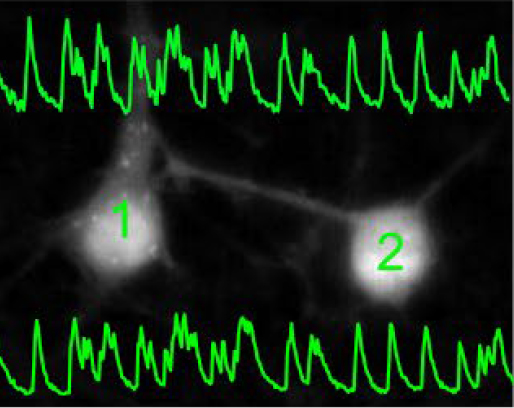
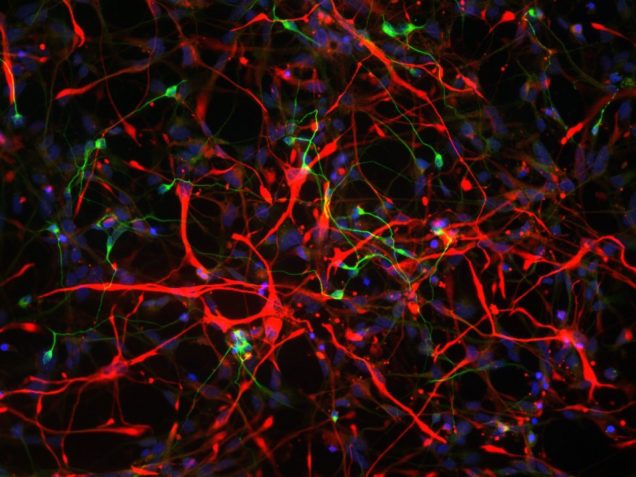
Stem cell imaging
Leading investigator Dr. Francesca Puppo |
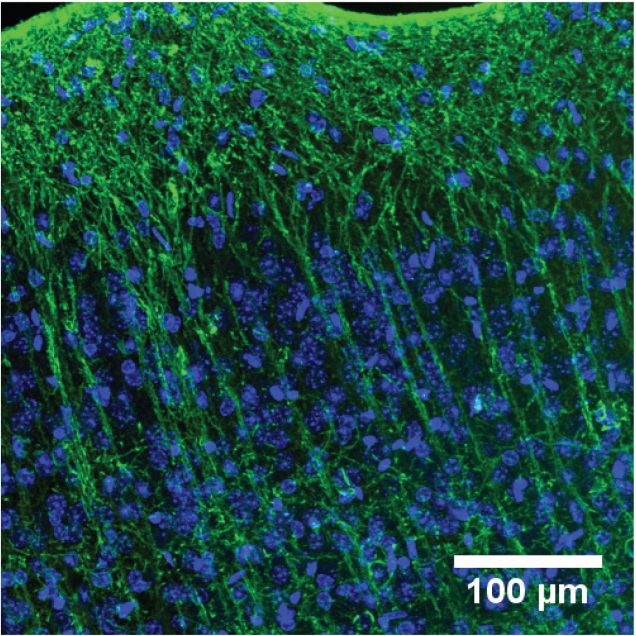
Traditionally, neuronal electrical properties have been evaluated using intracellular electrophysiology, which is low-throughput and labor intensive. Recent advances in optical microscopy and optogenetics offer a new experimental paradigm – “all-optical electrophysiology” – where optical voltage sensors and optogenetic actuators are combined for all optical stimulation and readout. Applied to human-induced pluripotent stem cells, all-optical electrophysiology opens unprecedented opportunities for high-throughput phenotyping of neurons and neuronal networks possessing unique genetic background of individual patients.
Contact Dr. Francesca Puppo at fpuppo@health.ucsd.edu |
Meet our collaborators:
 Dr. Alysson Muotri |
 Dr. Ole Andreassen |
 Dr. Srdjan Djurovic |
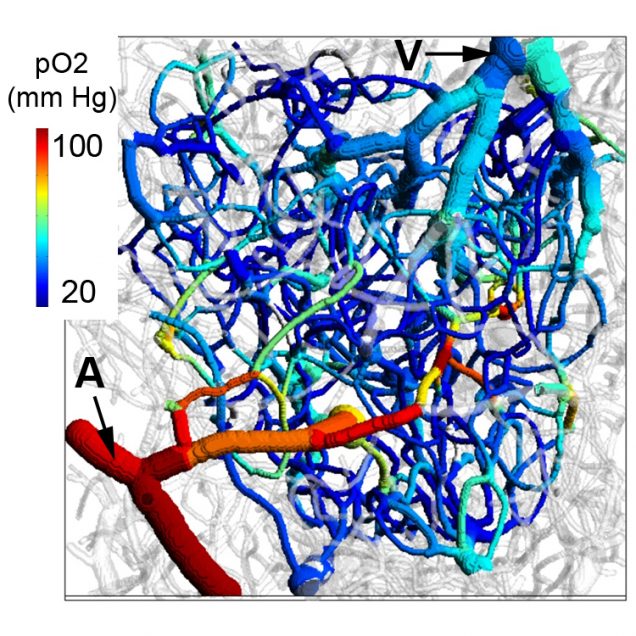
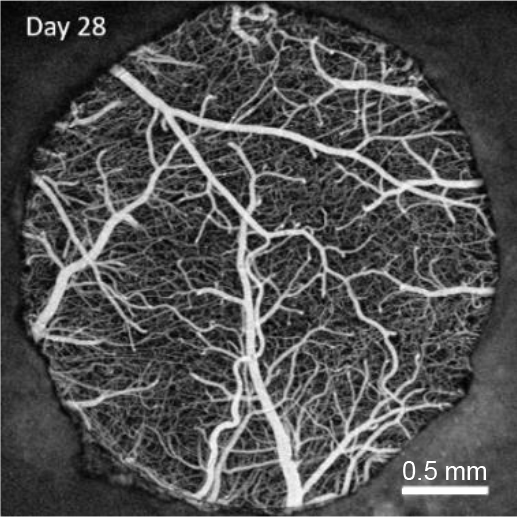
Oxygen imaging
Leading co-investigator Dr. Natalie Fomin |
Leading co-investigator Dr. Philipp Mächler |
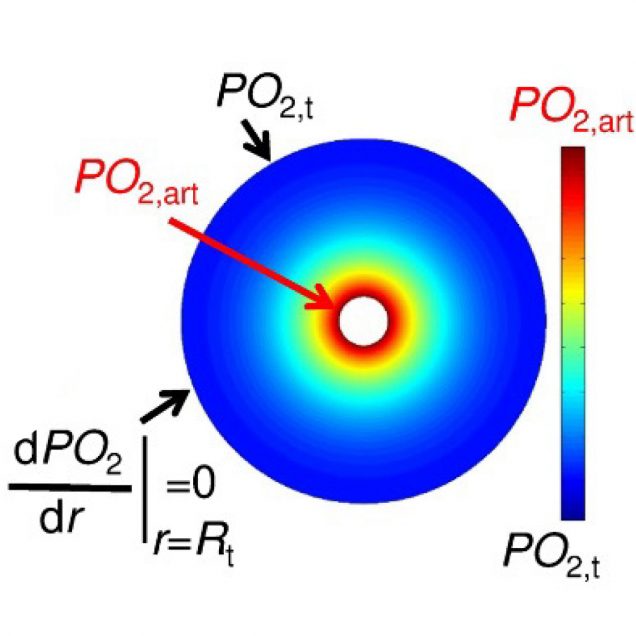
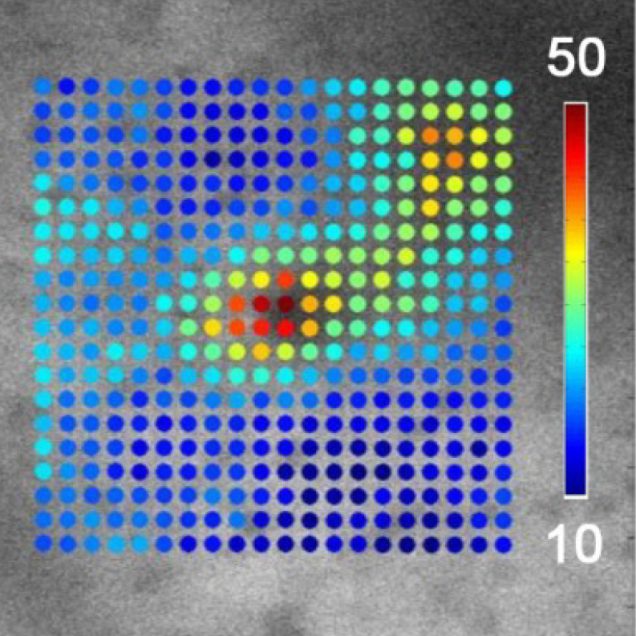
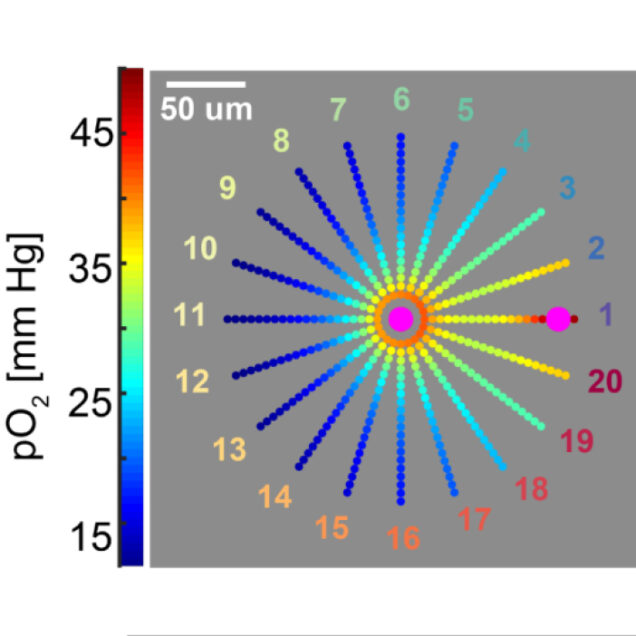
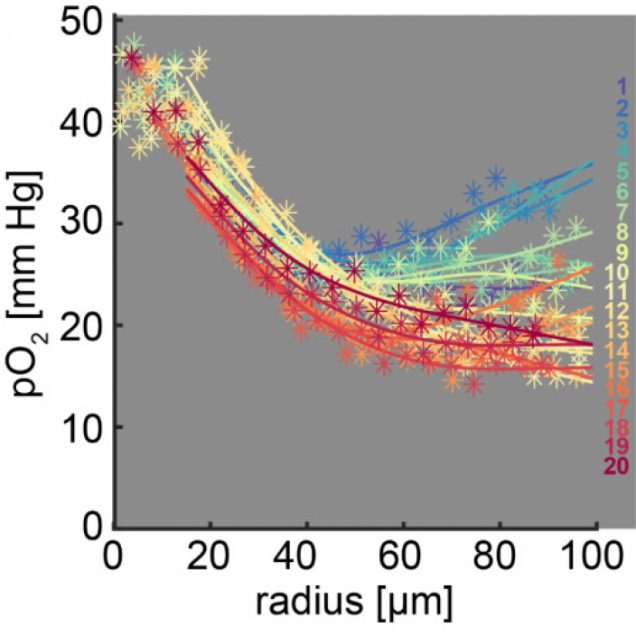
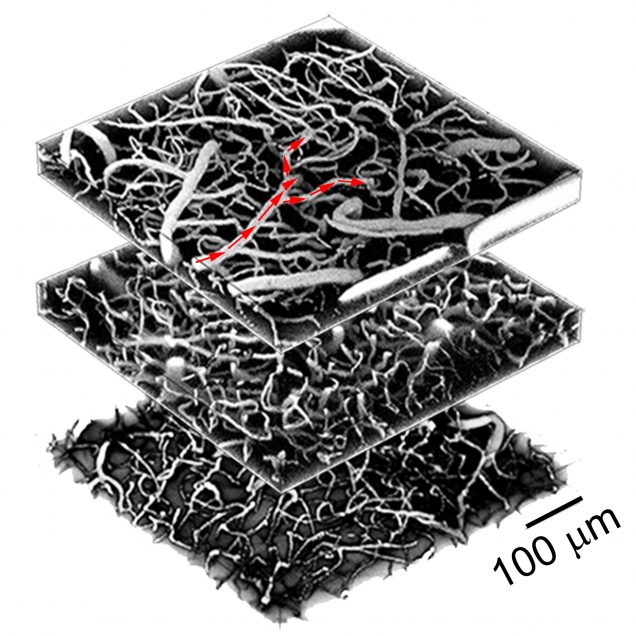
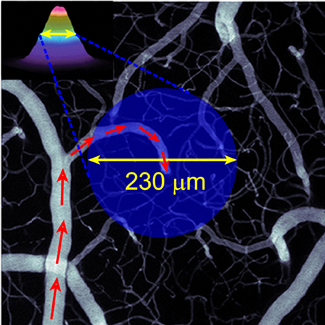
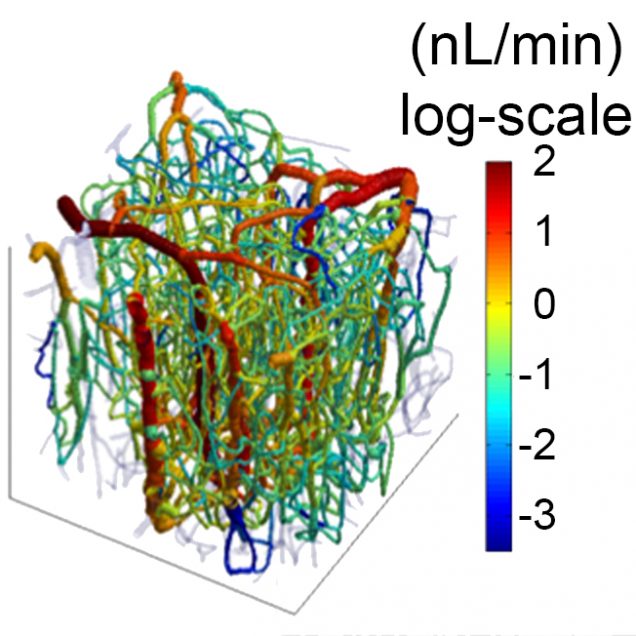
Recently, our ability to measure O2 with microscopic resolution has been revolutionized by the arrival of specific optical probes excitable in the two-photon regime and the development of two-photon phosphorescence lifetime microscopy (2PLM). 2PLM allows measurement of both intravascular and tissue partial pressure of O2 (pO2) with unprecedented spatial resolution and is well suited to imaging of pO2 changes at the baseline as well as during functional activation. 2PLM also offers a novel method for quantitation of CMRO2 based on pO2 gradients immediately surrounding diving arterioles.
Contact Dr. Natalie Fomin-Thunemann at n.fomin87@gmail.com or Dr. Philipp Mächler at philipp.maechler@outlook.com |
Meet our collaborators:
 Dr. Sava Sakadzic |
 Dr. David Boas |
 Dr. Sergei Vinogradov |
 Dr. Anders Dale |
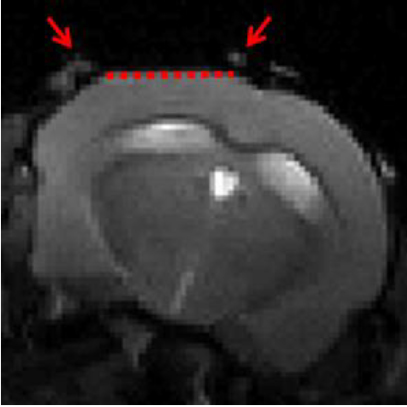
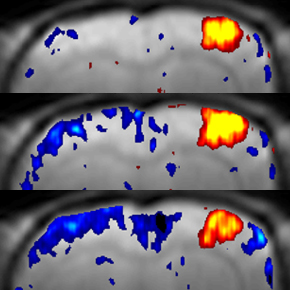
Awake mouse fMRI
Leading co-investigator Patrick Doran |
Leading co-investigator Patrick Doran |
Noninvasive imaging technologies such as fMRI are widely used to investigate the function of the human brain. However, interpretation of these macroscopic signals in terms of the underlying microscopic physiology, such as electrical activity of single neurons and hemodynamic activity of single blood vessels, is still under investigation. Noninvasive imaging in animals can play a critical role in physiological underpinning and data-driven modeling of human noninvasive signals, in particular when both micro- and macroscopic measurements are achieved in the same subject under analogous experimental conditions. To this end, we perform fMRI in awake mice implanted with chronic glass windows that provide optical access for micro- and “mesoscopic” optical imaging as well as for photostimulation.
Contact Dr. Philipp Mächler at philipp.maechler@outlook.com or Patrick Doran at pdoran@bu.edu |
Meet our collaborators:
 Dr. Sava Sakadzic |
 Dr. Michèle Desjardins |
 Dr. Joe Mandeville |
 Dr. Rick Buxton |
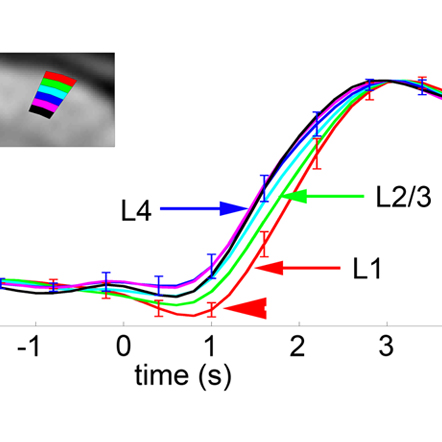
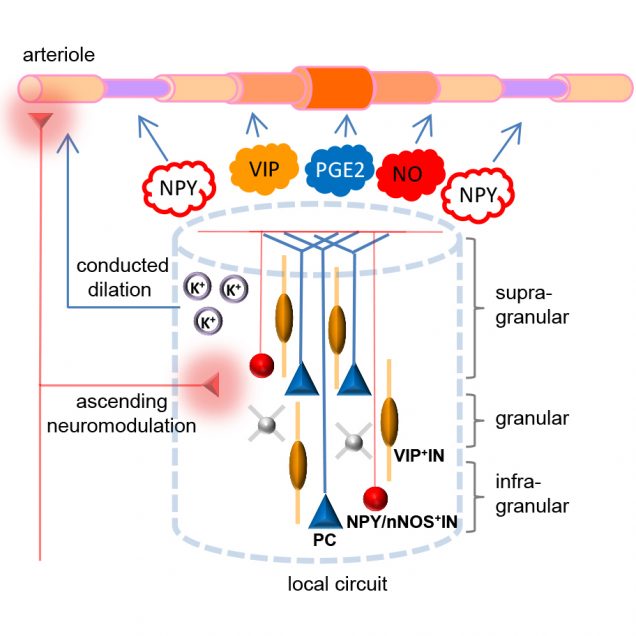
Cell-type-specific neurovascular coupling
Leading investigator Dr. Martin Thunemann |
Identification of the cellular players and molecular messengers that communicate neuronal activity to the vasculature driving cerebral hemodynamics is important for the basic understanding of cerebrovascular regulation and for interpretation of fMRI signals. A growing body of experimental evidence indicates that an increase in cerebral blood flow in response to increased neuronal activity (a.k.a. “functional hyperemia”) under healthy conditions is largely driven by vasoactive messengers related to neuronal signaling. We combine two-photon single-vessel measurements of vasodilation with optogenetic stimulation to study neurovascular regulation with high level of specificity and microscopic resolution.
Contact Dr. Martin Thunemann at martin@thunemann.de |
Meet our collaborators:
 Dr. David Kleinfeld |
 Dr. Martin Lauritzen |
 Dr. Sava Sakadzic |
 Dr. David Boas |