News
Open source mesoscope for imaging of two fluorophores and quantitative hemodynamics
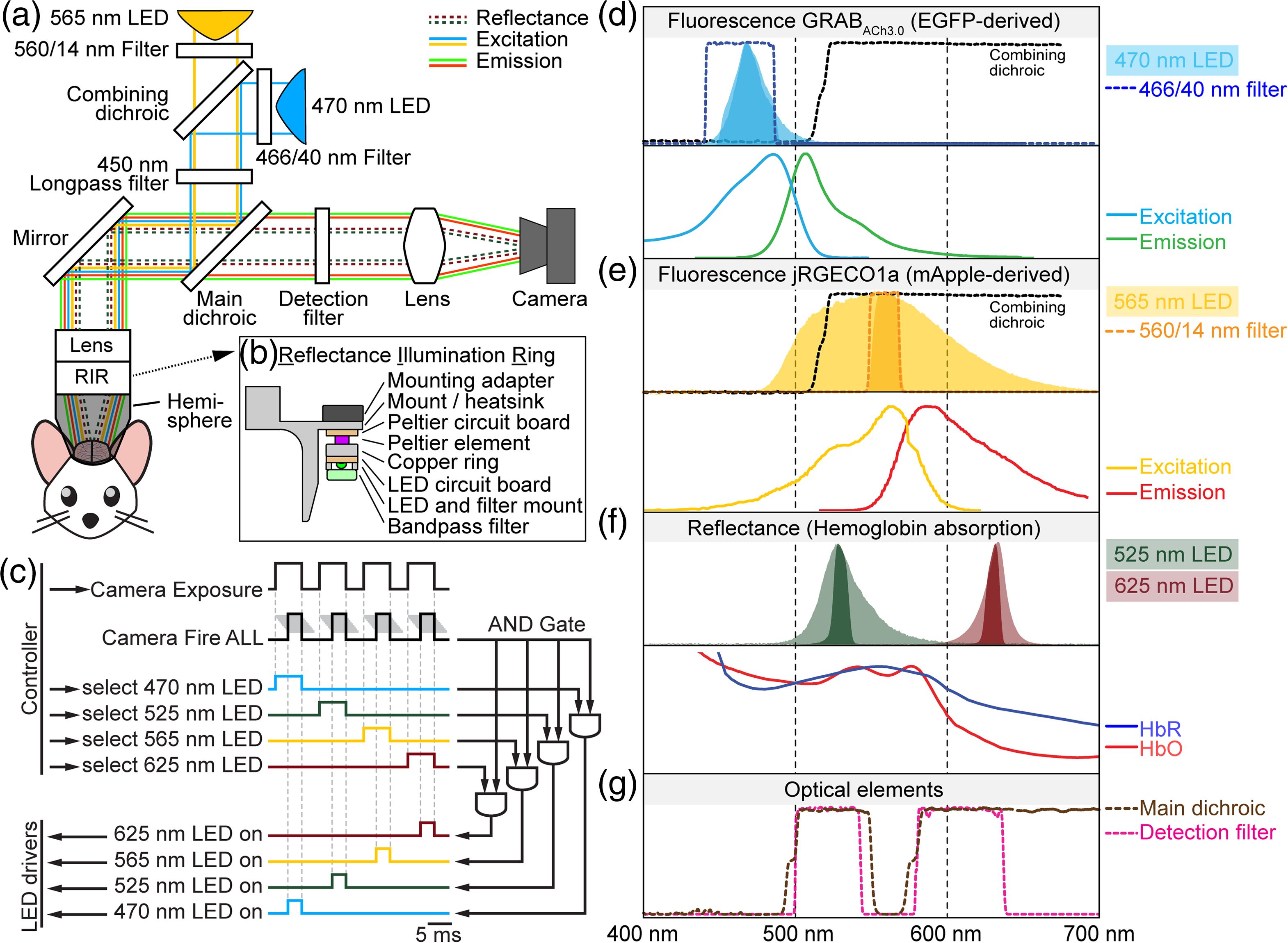 Wide field microscopy of the entire dorsal part of the mouse cerebral cortex enables large-scale imaging of spontaneous and evoked neuronal activity. This type of microscopy is perfectly suited to study interactions between cortical regions, particularly when cellular resolution is not required. Thanks to recent advances in molecular engineering, we have an array of fluorescent probes reporting different aspects of neuronal activity including changes in neuronal intracellular calcium concentration and release of specific neurotransmitters such as acetylcholine (ACh). These probes have different spectral variants allowing color multiplexing. Neuronal activity in vivo is coupled with changes in blood volume and hemoglobin oxygenation. These “hemodynamic” changes produce unwanted darkening of fluorescence signals due to light absorption by oxy- and deoxyhemoglobin (Hb and HbO), and simultaneous quantification of changes in hemoglobin concentration and oxygenation allows to correct this artifact. Furthermore, simultaneous imaging of neuronal activity and hemodynamics is needed for neurovascular studies.
Wide field microscopy of the entire dorsal part of the mouse cerebral cortex enables large-scale imaging of spontaneous and evoked neuronal activity. This type of microscopy is perfectly suited to study interactions between cortical regions, particularly when cellular resolution is not required. Thanks to recent advances in molecular engineering, we have an array of fluorescent probes reporting different aspects of neuronal activity including changes in neuronal intracellular calcium concentration and release of specific neurotransmitters such as acetylcholine (ACh). These probes have different spectral variants allowing color multiplexing. Neuronal activity in vivo is coupled with changes in blood volume and hemoglobin oxygenation. These “hemodynamic” changes produce unwanted darkening of fluorescence signals due to light absorption by oxy- and deoxyhemoglobin (Hb and HbO), and simultaneous quantification of changes in hemoglobin concentration and oxygenation allows to correct this artifact. Furthermore, simultaneous imaging of neuronal activity and hemodynamics is needed for neurovascular studies.
In this paper, we describe an open-source instrument that allows concurrent imaging of red and green fluorescence as well as hemodynamic imaging and quantification of Hb/HbO changes. The two-channel fluorescence imaging is performed in the epi-illumination mode. For absorption (hemodynamic) imaging, we use LEDs of two colors printed on a ring around the objective. During data acquisition, we strobe all four light sources for near-simultaneous acquisition of two fluorescence and two absorption time series. The LED ring serves as a wide base for a 3D-printed conical frustum that is placed in between the objective and a curved “crystal skull” cranial window. This design avoids unwanted visual stimulation from the strobing lights and provides diffuse, reflection-free illumination of the curved glass for absorption imaging. A single CMOS detector is used for detection of fluorescence and absorbance. The acquisition is controlled by a Matlab code interfacing with a National Instruments DAQ device.
Meet first author Patrick Doran |
Meet senior author Dr. Martin Thunemann |
Widefield in vivo imaging system with two fluorescence and two reflectance channels, a single sCMOS detector, and shielded illumination
Patrick R. Doran et al. Contact Patrick Doran at pdoran@bu.edu or Dr. Martin Thunemann at martinth@bu.edu |
Sensing neuronal activity in transplanted cortical organoids
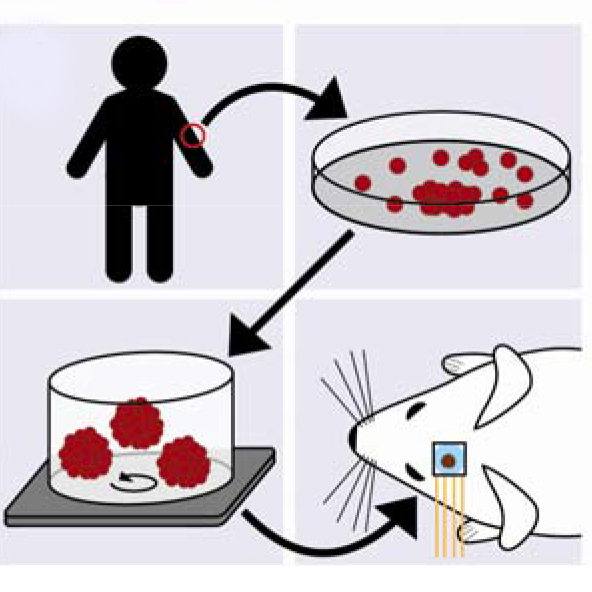
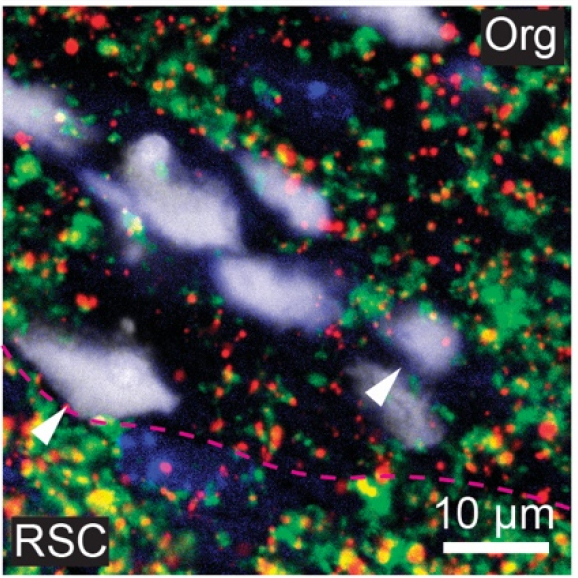
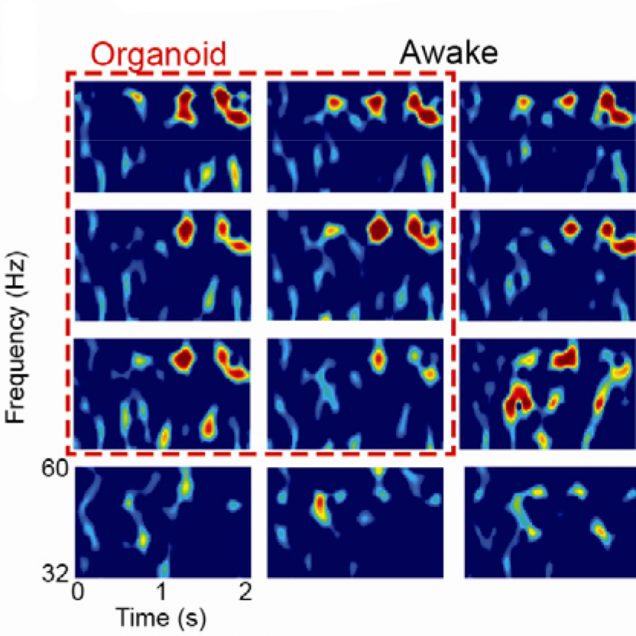
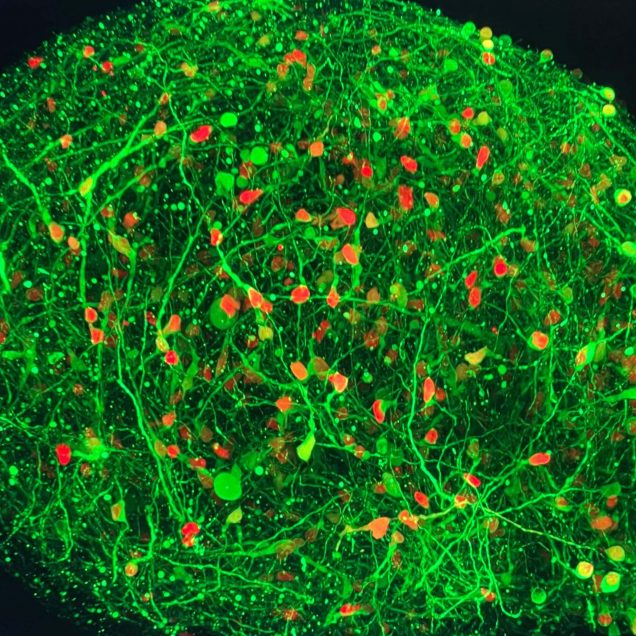
Cortical organoids are rapidly advancing as models of human brain development and disease and offer promise as neural prosthetics to restore lost or degenerated brain regions. However, due to limitations in longitudinal recording technologies, organoids’ beneficial abilities to functionally connect with host cortex upon implantation and respond to external sensory stimulation have yet to be demonstrated. In our new study in collaboration with the Kuzum and Muotri labs at UCSD, we established a novel paradigm combining transparent graphene electrode arrays and two-photon microscopy for longitudinal, multimodal monitoring of organoids implanted in mice cortices. Over the course of eleven weeks, we recorded local field potentials and multi-unit activity (MUA) from organoids in response to visual stimulation and during spontaneous activity. Examining the electrophysiological responses to visual stimulation, we found that the evoked responses in organoids matched that of the surrounding cortex, suggesting functional connectivity had formed between organoids and mouse tissue. In further support of functional connectivity, we observed increases in gamma and MUA power and phase locking of MUA to local field potentials following visual stimulation. Two-photon microscopy confirmed functional vascularization of the organoids. Using post-mortem immunohistochemistry, we observed synaptic connectivity and morphological fusion between organoids and host mouse cortices. Our novel, multimodal recording setup revealed the first demonstration of organoids functionally connecting to mouse visual cortical networks and offers a unique platform through which researchers can study the potential of organoids to restore damaged brain regions upon integration.
Meet first co-author Maddie Wilson |
Meet first co-author Dr. Martin Thunemann |
Multimodal monitoring of human cortical organoids implanted in mice using transparent graphene microelectrodes reveal functional connection between organoid and mouse visual cortex
Madison N. Wilson, Martin Thunemann, et al. Contact Dr. Martin Thunemann at martinth@bu.edu or Madison Wilson at m6wilson@eng.ucsd.edu |