Differential aberration imaging
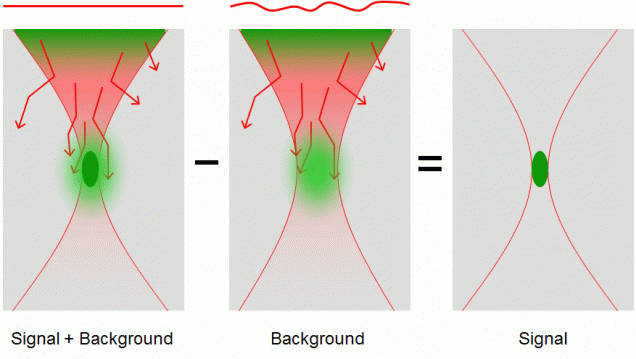
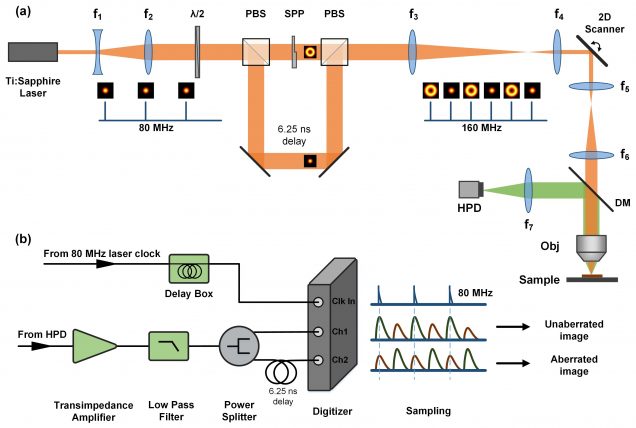
When a nonlinear microscope such as a two-photon excited fluorescence (TPEF) or second-harmonic generation (SHG) microscope is used to image thick tissue, the scattering of the excitation beam can provoke a loss of signal and an increase in out-of-focus background. We have alleviated the problem of increased background by developing a nonlinear microscope based on a novel contrast mechanism called differential aberration imaging (DAI. The principle of DAI is to introduce a switchable aberrating element in the excitation beam path. When the aberrating element is switched off, the microscope operates in a conventional manner, producing images that contain both signal and background. When the aberrating element is switched on, the extraneous aberrations in the excitation beam provoke a severe quenching of the signal, and the microscope reveals only background. A simple subtraction of an aberrated from an unaberrated image then results in an essentially background-free image that contains only signal. This technique works for any scanning microscope based on nonlinear contrast. For example, DAI was demonstrated with TPEF microscopy using as an aberrating element a deformable mirror provided by Boston Micromachines Corporation. More recently, instantaneous DAI has been achieved using temporal multiplexing with no moving parts.

- S. Xiao and J. Mertz, “Contrast improvement in two-photon microscopy with instantaneous differential aberration imaging”, Biomed. Opt. Express 10, 2467-2477 (2019). link
- A. Leray, K. Lillis, J. Mertz, “Enhanced background rejection in thick tissue with differential-aberration two-photon microscopy”, Biophys. J. 94, 1449-1458 (2008). link
- A. Leray, J. Mertz, “Rejection of two-photon fluorescence background in thick tissue by differential aberration imaging”, Opt. Express 14, 10565-10573 (2006). link