Retroillumination corneal imaging
The cornea hosts a number of remarkably transparent cells and microstructures. While their transparency is a boon for vision, it is also a detriment to their non-invasive observation (i.e. for diagnostics). Sensitive imaging methods are required to collect the minuscule amount of light scattered off corneal microanatomy and separate it from the much stronger reflection occurring at the air-cornea interface.
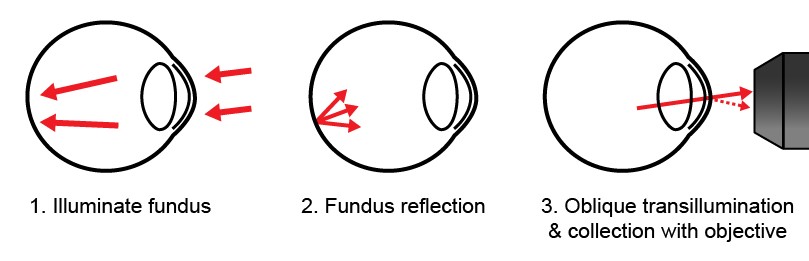
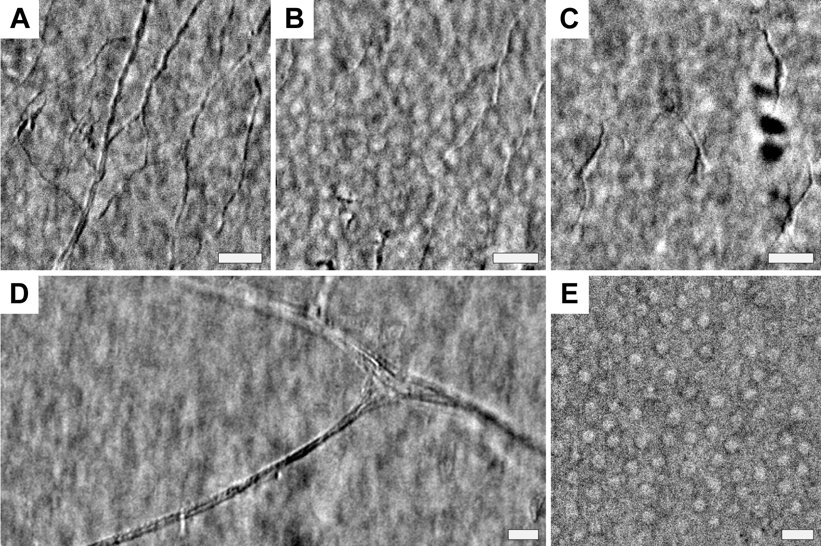
In the lab, we have developed a new corneal imaging approach based instead on transmitted light. The key idea is to use the retina as a diffuse back-reflector, thereby folding the light path of a widefield transmission microscope into one which requires access to only one side of the cornea (similar to Oblique Back-Illumination Microscopy). To maximize back-reflection efficiency, we use near-infrared light, which is only weakly absorbed and virtually undetectable to the subject. Additionally, we implement asymmetric illumination, a well-established method for enhancing intrinsic phase-gradient contrast. The technique produces micron-scale lateral resolution images across a large 1 mm diagonal field of view in the central cornea. We can routinely image cells in the corneal epithelium, the subbasal nerve plexus, large stromal nerves, dendritic immune cells, endothelial nuclei, and the anterior crystalline lens fibers and nuclei. Potential clinical applications include quantification of nerve density for disease monitoring and microbial keratitis diagnostics.
- T. D. Weber, J. Mertz, “In vivo corneal and lenticular microscopy with asymmetric fundus retroillumination”, Biomed. Opt. Express 11(6), 3263-3273 (2020). link