Atmospheric Airglow
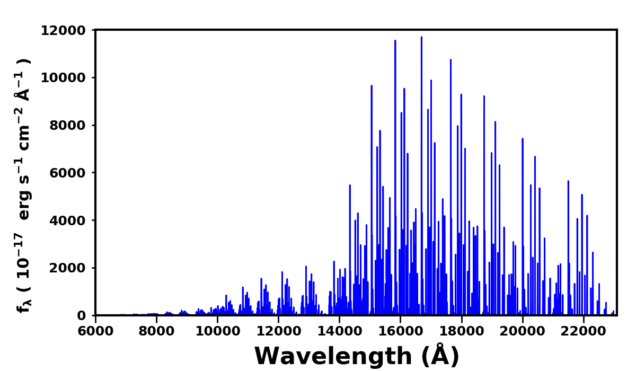
The Earth’s atmosphere absorbs and emits radiation. The most significant emission in the optical and near-infrared (NIR) is due to the ro-vibrational transitions of the OH molecule. OH is created in the ozone hydration process, H + O3 –> OH + O2, and is formed in a vibrationally excited state. The vibrationally excited molecule is relaxed through collisional quenching and radiation emission. The figure below is a Sloan Digital Sky Survey (SDSS) Baryon-Acousitc Oscillation Spectroscopic Survey (BOSS) sky spectrum which has been annotated to highlight the OH transitions.
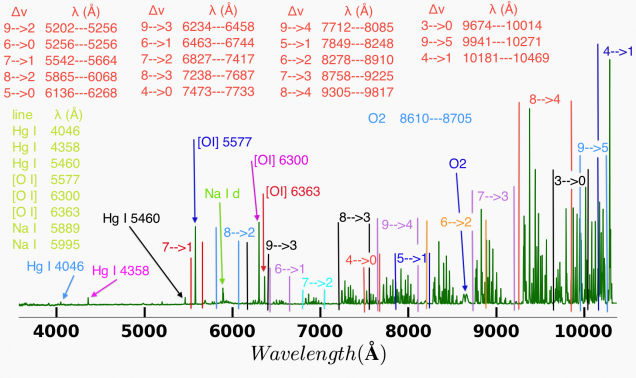
The OH spectrum has a band head structure show in the figure below. The excited vibrational states of the OH molecule are accompanied by a range of rotational states, and the vibrational transitions can be accompanied by rotational changes in state. The change in rotational quantum number can be -1, 0, or +1 giving rise to the R, Q, and P branches of the OH emission spectrum. The energy levels of the OH molecule also experience spin orbit splitting leading to the F1 and F2 electronic states. The energy levels of the OH molecule are also inverted because the higher total angular momentum of the F1 electronic states are lower in energy than the F2 states.
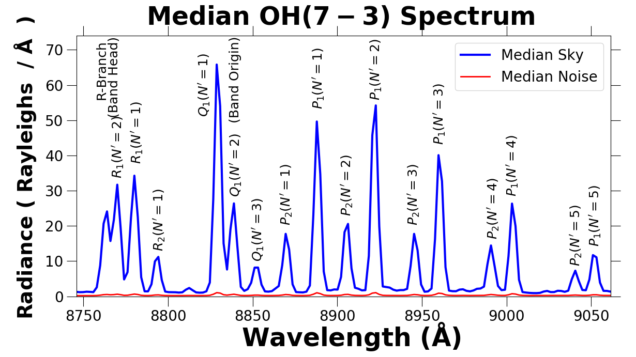
The OH spectrum increase in intensity at the lower vibrational transitions, and peaks around 17,000 A. The spectrum below is a model OH spectrum.