COVID-19 Effect on Pharmaceutical R&D Supply Chains
Researchers: Dave Weidman, Yuanfei Zang, Canan Gunes Corlu, Yujue Tan, and John Maleyeff
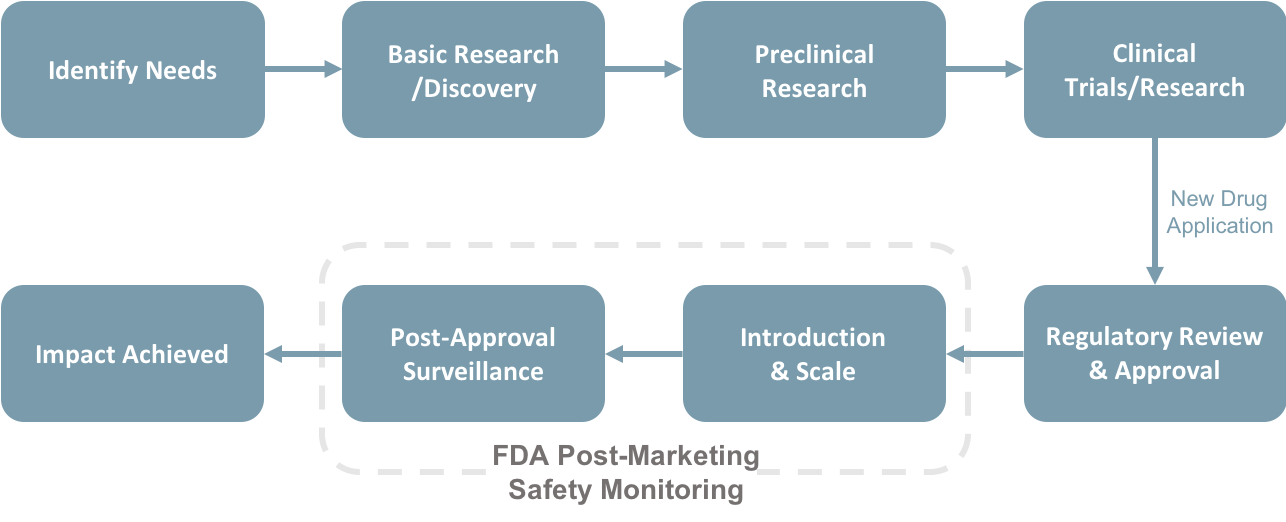
Pharmaceutical/biotechnology (Pharma/Bio) companies face risks associated with their Research and Development (R&D) supply chains that need to be constantly monitored and managed. Under normal circumstances, managing these risks can be a substantial challenge. When you factor in the COVID-19 pandemic, some risks become exacerbated. Pharma/Bio companies and their suppliers must now deal with employee safety and talent management issues; the shift to a technology-based remote working model; the reallocation of R&D resources to COVID-19 vaccines and therapies, etc.
The aim of the project is to create a timely analysis of the impacts that the COVID-19 pandemic has had on the Massachusetts’ Pharma/Bio industry by conducting an online survey. This approach provides an effective way to understand how risks associated with the Pharma/bio companies R&D supply chain (e.g., raw material suppliers, external test labs, information capture and flow, venture capital funding, etc.) have been affected by the pandemic. One of our focuses will be on how these companies have/are applying Enterprise Risk Management (ERM) methodologies in conjunction with Supply Chain Management (SCM) modeling methods to address the COVID-19 crisis. Results of the project will be incorporated into future classroom discussions.
Image by Yuanfei Zang