APOE2
Project Title: Investigation of therapeutic targets mimicking APOE ε2 protective mechanisms in Alzheimer Disease
Project Goals: Identify mechanisms underlying the protective effect of APOE ε2 in Alzheimer Disease using genetic association and experimental approaches in brain tissue and neuronal cell lines.
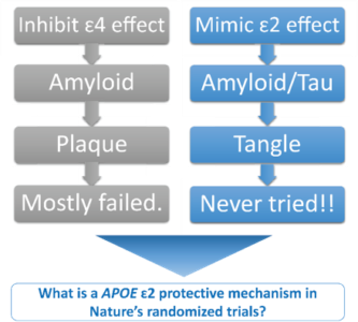
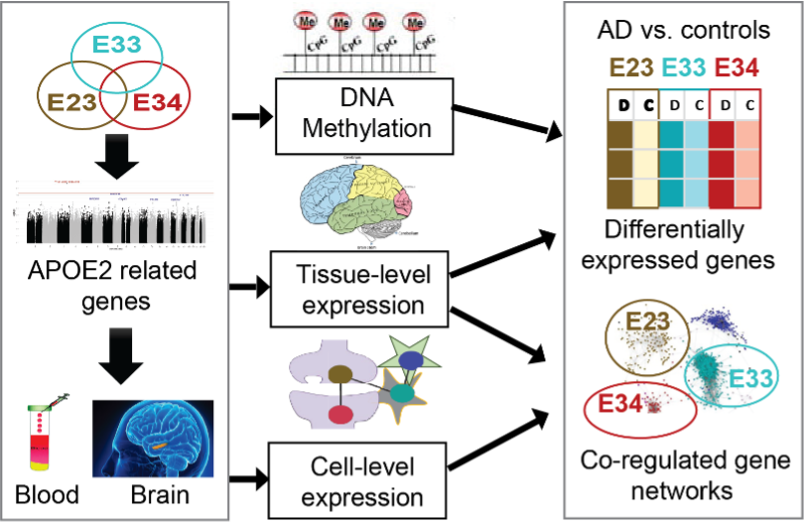
Project Summary: APOE is the major risk gene for late onset Alzheimer’s disease. In US and international genetics consortium, Dr. Jun led and conducted innovative analysis approaches to identify distinct genetic signatures contributing to Alzheimer’s disease risk in APOE4-positive vs. APOE4-negative subgroups. This effort has reported one of the most striking discoveries in the Alzheimer’s disease genetics field that genetic variants in the MAPT/KANSL1 locus are strongly associated with Alzheimer’s disease among subjects who do not carry the APOE4 allele of the APOE gene (Jun et al. 2015). This is an important breakthrough in Alzheimer’s disease genetics, because for the first time APOE genotypes modulate effects of MAPT locus on Alzheimer’s disease risk. The follow-up analysis of the initial report (Jun et al. 2016) using APOE2-carrier subjects by separating from the APOE4-negative subgroup has discovered genetic variants in PPP2CB with strong genetic interaction with the APOE2 allele, where PPP2CB encodes a catalytic subunit of protein phosphatase 2A that is responsible about 71% of tau phosphatase activities in human brain (reducing tau phosphorylation). After the first report of APOE as the major genetic risk factor for Alzheimer’s disease in 1993, therapeutic concepts targeting APOE primarily involve in correcting or inhibiting the APOE4 effect, the risk factor for Alzheimer’s disease. For the first time, beyond APOE4, Dr. Jun has developed a novel therapeutic concept for Alzheimer’s disease that mimics the APOE2 protective mechanism. A five-year grant proposal based on this innovative idea was funded by the NIH/NIA. In this project, we will perform functional experiments for connecting APOE2 protective mechanism to tau and conduct in vitro proof-of-concept experiments for developing new drugs for Alzheimer’s disease based on APOE-mediated protective mechanisms.