Projects
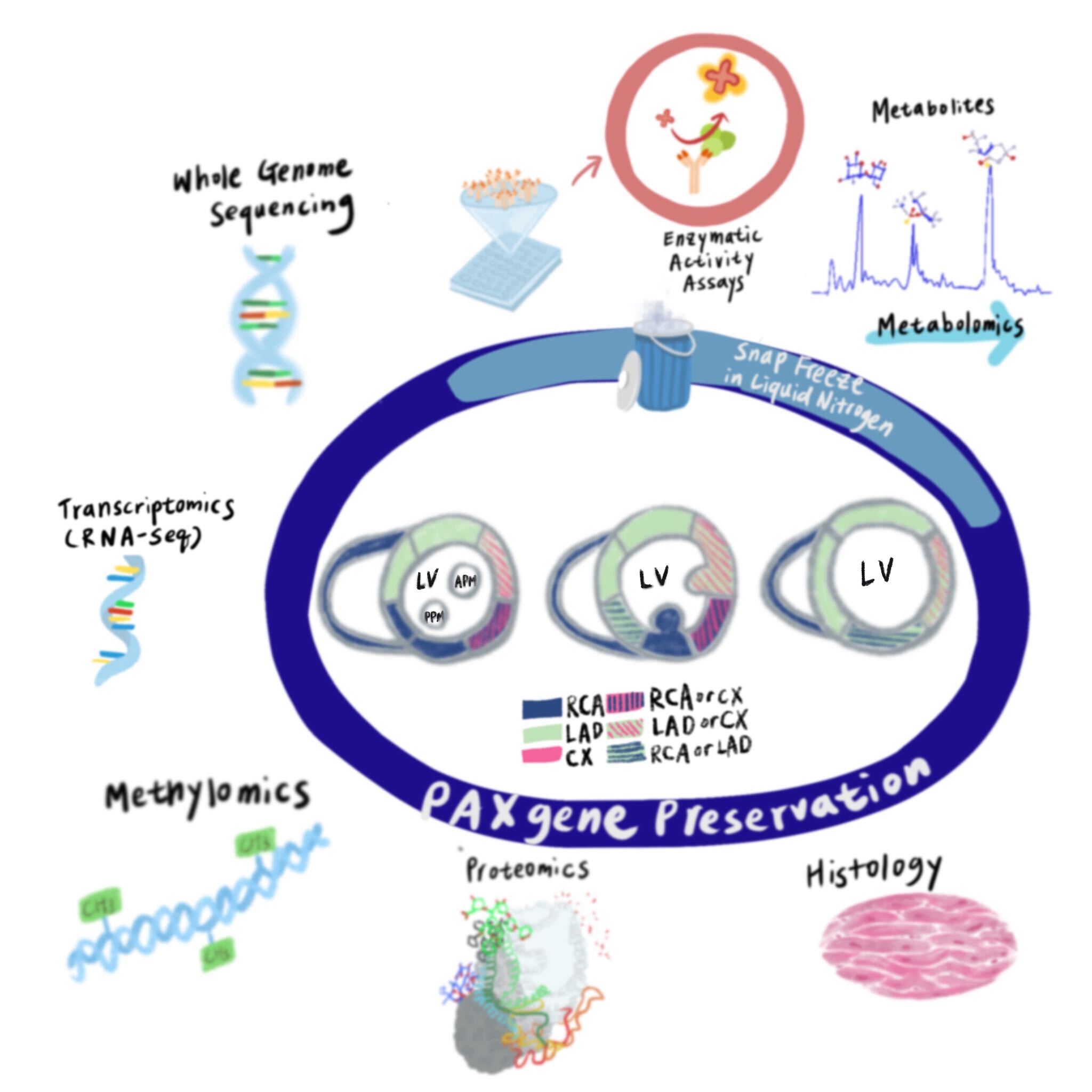
Cardiovascular Biobanking for Multiomics
The effect of cardiovascular risk factors and disease on human cardiovascular tissue cell types and molecular signatures is largely unknown. Current knowledge of human cardiovascular tissue biology is based on limited chamber biopsies/postmortem sampling in specific diseases, limiting generalizability to other cardiovascular diseases. Our understanding of human cardiovascular pathophysiology is largely based on circulating markers and cells, which likely do not reflect cardiovascular tissue biology. We are generating a novel cardiovascular biobank consisting of whole human hearts and proximal vessels preserved to maintain the spatial resolution for multiomics interrogation, linked to robust antemortem clinical and circulating omics data. Deep phenotyping of cardiovascular tissue across the entire organ will facilitate high-fidelity, expansive systems-biology profiling that can be paired with rich, longitudinal clinical, imaging, biomarker, and genetic data.
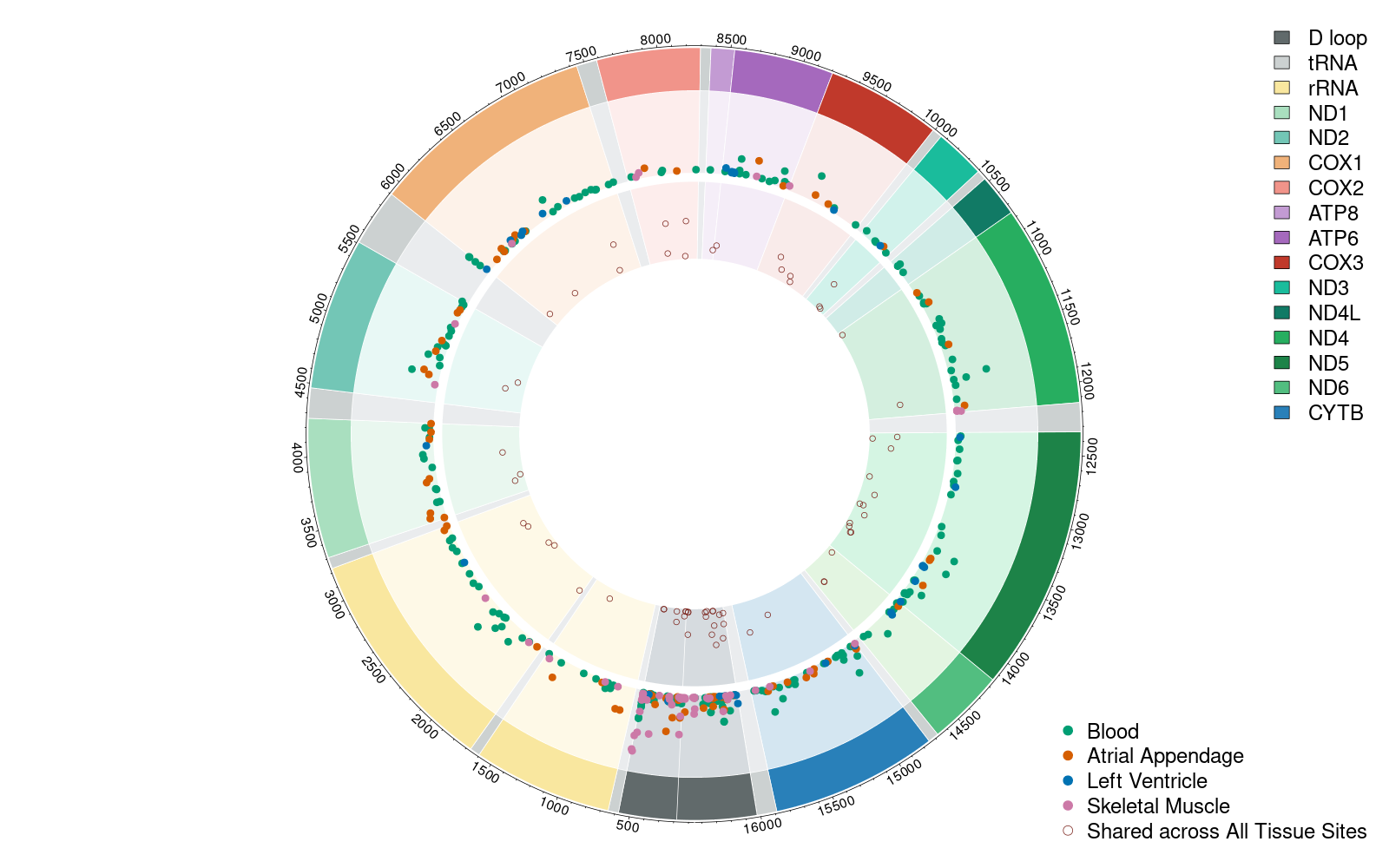
Mitochondrial Genetics in Cardiovascular Pathophysiology
An overarching goal of ours is to identify the mechanisms for how mitochondrial genetic variation contributes to the development of cardiovascular diseases. We are creating a number of software packages to enhance our ability to delineate pathogenic from benign mitochondrial genetic variants, identify mitochondrial genetic variants from RNA-seq datasets, and enable the investigation of mitochondrial-nuclear genetic interactions. We have several ongoing investigations on the relation of mitochondrial genetic variants with cardiovascular risk factors and disease, and clinical measures of cardiac structure and function at the population level.
We are also developing high-throughput methods to evaluate mitochondrial function in biobanked clinical samples from large human cohort studies. Such methods will allow us to evaluate whether differences in mitochondrial function mediate the effects of mitochondrial genetic variants with cardiovascular disease in humans. The tools we are developing can be readily applied to many other cell types and phenotypes of interest as mitochondria play central roles in cellular function and are implicated in many human diseases.
Mitochondrial Disease Modeling Using Human Induced Pluripotent Stem Cells (iPSCs)
We have begun to develop lines of investigation using human iPSCs to comprehensively evaluate mitochondrial genetics and physiology in human cardiovascular cells. In doing so, we utilize the institutional Framingham Heart Study iPSC biobank and are actively creating a biobank of iPSCs generated from blood samples donated by patients with mitochondrial and metabolic diseases. A greater understanding of the contribution of mitochondrial genetic variation in human cardiomyocytes will facilitate the development of targeted interventions and therapeutics designed to modulate mitochondrial function and ultimately improve cardiovascular outcomes in patients with mitochondrial disease and more prevalent forms of cardiovascular diseases.