Our research
The central question that drives our research is: How do the structure, stability and misfolding of antibody light chains contribute to AL amyloidosis pathology?
AL amyloidosis is a systemic amyloidosis caused by aggregation of antibody light chains to form amyloid fibrils. These light chains are secreted by plasma cells, which are specialized B cells that secrete antibodies, part of the adaptive immune system. Each plasma cell produces a unique light chain sequence, which is selected for binding to an antigen in a process central to the adaptive immune system. In AL amyloidosis, a single light chain sequence is produced from a clonal population of plasma cells in the bone marrow, and aggregates in other tissues around the body. Most light chain sequences are not associated with amyloid formation, even when secreted at high levels from cancerous plasma cells in multiple myeloma, and AL amyloidosis patients tend to have only moderate levels of their clonal antibody. It seems that the specific sequence of the light chain – and therefore the protein’s structure and dynamics – determines its propensity to aggregate.
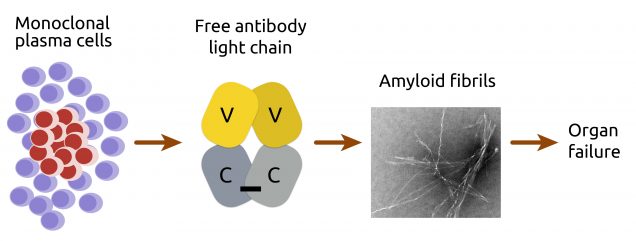
We study how these differences lead to the broad range of different pathologies that affect AL amyloidosis patients. By identifying factors that are associated with amyloidosis, we aim to design therapies that can prevent amyloid pathology and treat patients.
Recent publications
Light Chain Stabilization: A Therapeutic Approach to Ameliorate AL Amyloidosis. Hemato (2021) Review of the potential utility of a stabilizer drug for AL amyloidosis. Pubmed DOI
Morgan GJ, Yan NL, Mortenson DE, Rennella E, Blundon JM, Gwin RM, Lin C-Y, Stanfield RL, Brown SJ, Rosen H, Spicer TP, Fernandez-Vega V, Merlini G, Kay LE, Wilson IA and Kelly JW (2019) Stabilization of amyloidogenic immunoglobulin light chains by small molecules. Towards a stabilizing drug for AL amyloidosis. Pubmed DOI
Rennella E, Morgan GJ, Kelly JW, Kay LE (2019) Role of domain interactions in the aggregation of full-length immunoglobulin light chains. Interactions within light chain dimers stabilize their structure and prevent aggregation. Pubmed DOI
Morgan GJ, Usher GA, Kelly JW (2017) Incomplete Refolding of Antibody Light Chains to Non-Native, Protease-Sensitive Conformations Leads to Aggregation: A Mechanism of Amyloidogenesis in Patients? Full-length light chains can misfold to a state with a non-native trans-proline in their constant domain. Pubmed DOI
Morgan GJ, Kelly JW (2016) The Kinetic Stability of a Full-Length Antibody Light Chain Dimer Determines whether Endoproteolysis Can Release Amyloidogenic Variable Domains. Full-length light chains are much less likely to aggregate than their isolated variable domains. Pubmed DOI