Our Research
Oral Squamous Cell Carcinoma
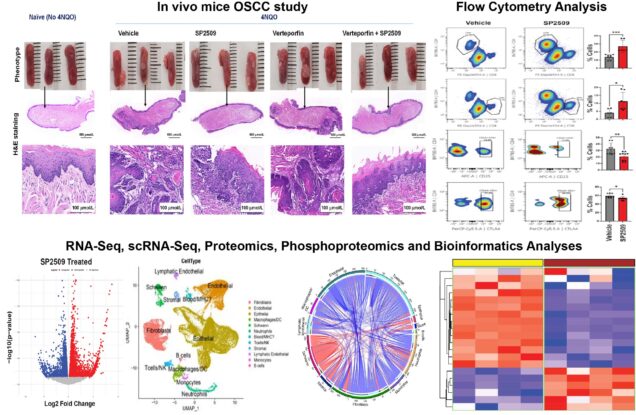
Lysine-specific demethylase 1 (LSD1) is a nuclear histone demethylase. LSD1 expression progressively increases with tumor grade and stage in clinical oral squamous cell carcinoma (OSCC). Our long-term goal is to evaluate LSD1 mechanism in progressive oral malignancy based on preliminary studies for therapeutic applications. We showed for the first time that blocking LSD1 inhibits preneoplasia, a feed-forward loop during the progression of dysplasia to OSCC. Interestingly, LSD1 inhibition attenuates tumorigenic signaling identified in two independent studies 1) proteomics analysis of Lsd1 knockout mice preneoplasia and 2) single-cell RNA-Seq analysis using LSD1 inhibitor. However, the mechanism of LSD1 function, its target cells in progressive oral malignancy, and how LSD1 promotes tumorigenic signaling remain unclear. The successful completion of the proposed project is expected to identify LSD1 and therapeutic application mechanisms in the this novel signaling axis. Finally, the study will determine if LSD1 has a role in immunotherapy resistance and whether pharmacological LSD1 inhibition can attenuate animal OSCC for a potential application in veterinary and human medicine. Overall, the study will have a broader impact on future translational studies in human preneoplasia. Thus, this study has a strong translational potential to identify LSD1 as a novel druggable target and its potential for anti-OSCC monotherapy and combination therapies including immune checkpoint inhibitor and radiation therapy.
Temporomandibular Joint Osteoarthritis
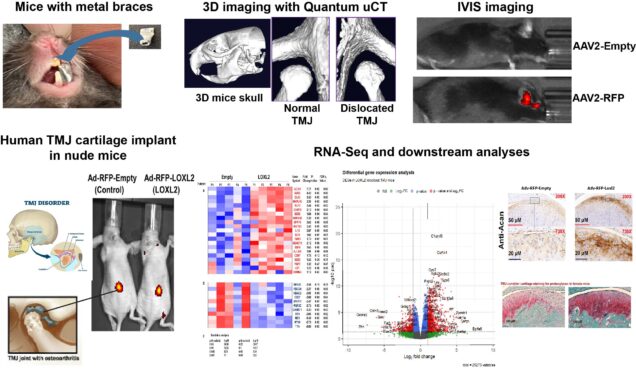
Temporomandibular joint (TMJ) disorders affect 5–12% of the United States population. The TMJ has fibrocartilage that exhibits functional and pathological differences in the development of OA compared to other joints, and this joint is less well studied. Treatment options remain limited, but limited anabolic and protective agents are being evaluated for their potential to regenerate TMJ cartilage. We recently reported that the lysyl oxidase like-2 (LOXL2), a copper-dependent amine oxidase, promotes chondrogenic differentiation and proteoglycan deposition during cartilage formation and maintenance. LOXL2 is expressed in regenerating cartilage during fracture healing and in human TMJ cartilage. Earlier studies supported by our R03 award (R03DE025274) showed that LOXL2 expression is elevated in OA-affected cartilage in the TMJ, compared to normal tissues, and that it co-localizes with the OA-related pro-inflammatory cytokine IL-1bin addition to anabolic factors. Importantly, LOXL2 overexpression upregulates anabolic proteins in human chondrocytes, supporting its critical role in chondrogenesis. LOXL2 knockout mice showed a reduction of these anabolic proteins. We also found that LOXL2: 1) promotes anabolic responses in TMJ cartilage of a chondrodysplasia (Cho/+) mouse model; 2) enriches gene sets related to extracellular matrix remodeling, increases proteoglycan deposition, and upregulates aggrecan gene expression in human TMJ chondrocyte implants in nude mice. In addition, we identified that LOXL2 inhibits pro-inflammatory signaling in chondrocytes. We hypothesize that LOXL2 is critical for maintaining TMJ fibrocartilage homeostasis, TMJ chondroprogenitor function, and epigenetic regulation. Although TMJ-related disorder is a major health and societal burden that lacks an FDA-approved therapy, few studies are in progress to identify new therapeutic strategies. Our preliminary data support that LOXL2 is critical for TMJ cartilage regeneration and potential therapeutic application.
Knee Osteoarthritis
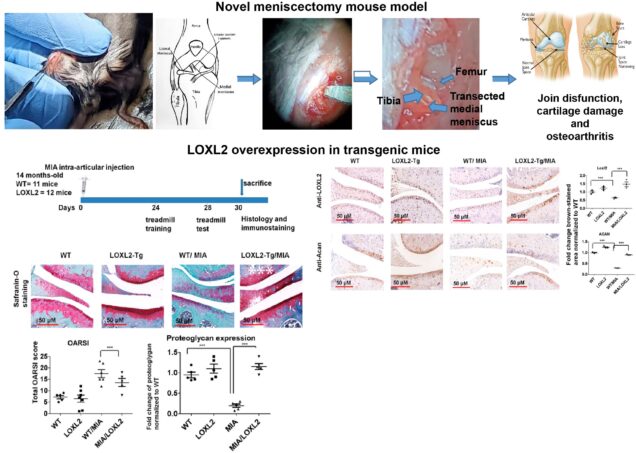
Cartilage has a limited potential for self-regeneration—any damage to cartilage results in structural, molecular, and functional changes in the knee joint. Early changes in cartilage and extracellular matrix are primarily asymptomatic, whereas it progresses towards knee joint dysfunction, pre-osteoarthritis (OA), and finally, OA. Thus, the identification of agents which promotes cartilage and its ECM are critical for the development of disease-modifying agent. We have uncovered potentially novel functions of LOXL2 in cartilage regeneration and restoring knee joint function. We showed that LOXL2 overexpression in human OA articular chondrocytes induces anabolic proteins expression while attenuating catabolic expression of MMP13 and NF-kB signaling. Conversely, LOXL2 knockout mice increase the severity of destabilized medial meniscus (DMM) -induced cartilage damage. LOXL2-overexpressing transgenic mice are protected against chemically-induced degenerative cartilage changes in the knee joint, restore treadmill running capability, and reduced allodynia compared to wild-type littermates. Interestingly, intra-articular injection of Adenovirus delivered LOXL2 protects knee joint function and alleviates cartilage degeneration. The gap in the knowledge exists in understanding 1) how LOXL2 function promotes cartilage regeneration and knee joint function and 2) LOXL2 regenerated cartilage has similar structural and functional properties to native cartilage. Based on preliminary studies, we hypothesize that LOXL2 promotes proteoglycan deposition during cartilage regeneration and restores knee joint function. Further, LOXL2, or its derivatives, promotes new cartilage with similar biomechanical, molecular, and functional properties to native cartilage, which has an impact for future translational applications. Our multidisciplinary approach is expected to establish if LOXL2 loss impairs, whereas LOXL2 gain regenerates cartilage and knee joint functions by specific mechanisms.
