Outpatient and Home-Use Clinical Trials
iLet™ Multi-PK Profiling Study
March 2019 – December 2019
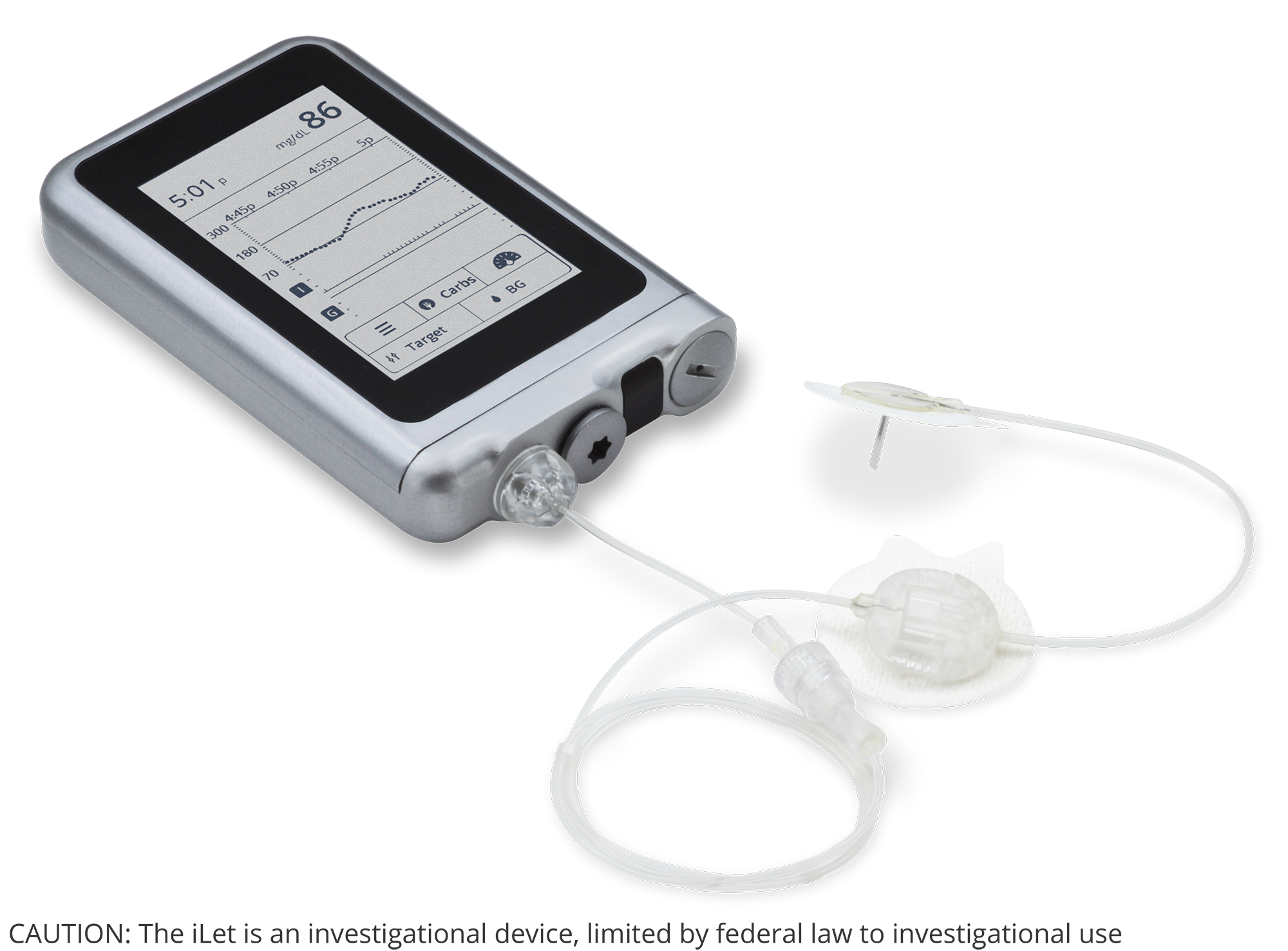
Three 7-day iLet experiments in adults with T1D
- Up to 30 subjects, each participating in three 7-day randomized cross-over treatment arms
- One week with Humalog, one week with Novolog, and one week with BioChaperone® Lispro (BC222)
- Must be ≥18 years old and remain within 250 miles of study base with no air travel
- Home use study: People with T1D engaged in usual daily work and leisure routine
- Realtime telemetric monitoring for hypoglycemia and hyperglycemia
Fiasp® with iLet™ PK Exploratory Study
March 2019 – May 2019

Two 7-day iLet experiments in adults with T1D
- Three cohorts, eight subjects per cohort, with each subject participating in two randomized cross-over treatment arms
- Treatment arm 1: 7 days on the iLet with Fiasp using usual pharmacokinetic (PK) settings
- Treatment arm 2: 7 days on the iLet with Fiasp using exploratory PK settings
- Three days in a supervised study setting, 4 days in a home-use setting
- Must live within 120 minutes of study base
- Must have a screening A1c within 6.5-9%
- Realtime telemetric monitoring for hypoglycemia and hyperglycemia
The Bionic Pancreas Multi-Center Study
June 2014 – April 2015

11-day experiments in adults with T1D
- Randomized cross-over design (11 days on bionic pancreas, 11 days on usual care)
- Home use study: People with T1D engaged in usual daily work and leisure routine
- Must live within 30 minutes, stay within 60 minutes of study base
- Sleep at home (with a designated contact), technical & medical support on call
- Telemetry to monitor wireless connectivity of pumps and CGM, and if the CGM reads < 50 mg/dL
- 40 adult subjects 18 years and older (440 bionic pancreas days)
2014 Summer Camp Study
July 2014 – August 2014

5-day experiments in pre-adolescents with T1D
- Randomized cross-over design (5 days on bionic pancreas, 5 days on insulin pump therapy)
- Integrated camp experience at Camp Joslin (boys) and Clara Barton Camp (girls)
- Point of care capillary BG checks during day and night
- Study staff and camp staff provide 24-hour coverage; round-the-clock telemetry to monitor wireless connectivity of pumps and CGM
- 7 boys, 13 girls (6-11 years old) with T1D (120 bionic pancreas days)
2013 Summer Camp Study
July 2013 – August 2013
5-day experiments in adolescents with T1D
- Randomized cross-over design (5 days on bionic pancreas, 5 days on insulin pump therapy)
- Integrated camp experience at Camp Joslin (boys) and Clara Barton Camp (girls)
- Point of care capillary BG checks during day and night
- Study staff and camp staff provide 24-hour coverage; round-the-clock telemetry to monitor glycemia
- 16 boys, 16 girls (12-20 years old) with T1D (160 bionic pancreas days)
Please visit our clinicaltrials.gov study record.
(complete, no longer enrolling)
The Beacon Hill Study
February 2013 – September 2013
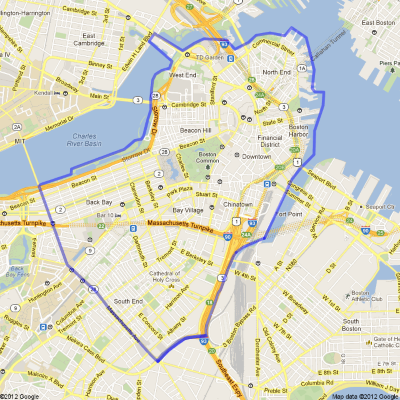
5-day experiments in adults with T1D
- Randomized cross-over design (5 days on bionic pancreas, 5 days usual care)
- Free run of Boston peninsula east of Mass Ave (three square mile area)
- Point of care capillary BG checks during day, 1:1 nursing
- Sleep in hotel with venous BG monitoring at night, 1:2 nursing
- 20 adult subjects 21 years and older (100 bionic pancreas days)
Please visit our clinicaltrials.gov study record.
(complete, no longer enrolling)

