Calponin (CaP)
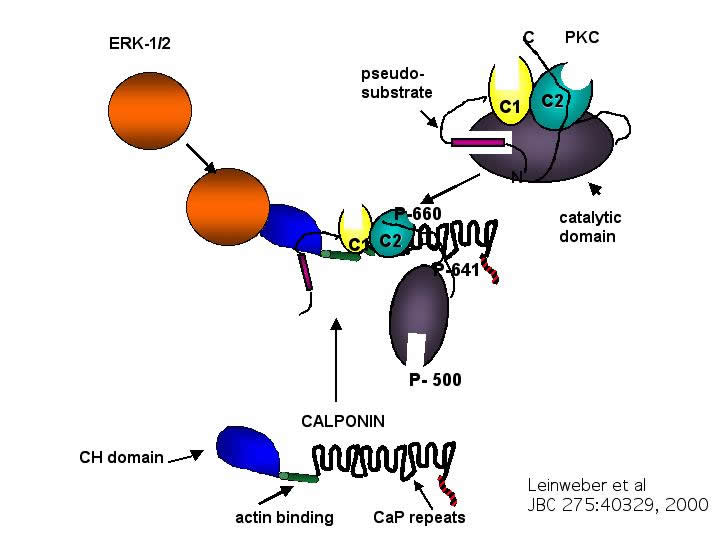
CaP is an actin binding protein that inhibits actomyosin ATPase activity in vitro. We have found that CaP, unlike other actin binding proteins which inhibit actomyosin ATPase activity (such as caldesmon and troponin), undergoes an agonist induced translocation from the cytoskeleton to the submembranous cortex. CaP co-immunoprecipitates with ERK1 and with PKC epsilon in ferret aorta homogenates, co-localizes in cells with ERK1 and PKC epsilon, and binds activated PKC epsilon in a gel overlay assay. We have speculated that CaP functions as an adaptor protein connecting the PKC cascade to the ERK cascade. The ERK-binding function of CaP is localized to its N-terminal CH domain. We have speculated that other CH domains may be ERK-binding domains. [Leinweber et al 1999]
We have found that calponin can interact with the regulatory domains of PKC epsilon and directly activate kinase activity in a lipid independent manner, as assayed by 32P incorporation into peptide substrates and by autophosphorylation experiments using a phospho-specific PKC antibody. These results, in combination with past studies, suggest that calponin has the potential to be an in vivo protein modulator of PKC activity. [Leinweber et al2000]