The dark side of light chains
The main focus of the lab is a disease called AL amyloidosis, which is caused when antibody light chain proteins form insoluble clumps called amyloid fibrils throughout the body.
Antibodies, otherwise known as immunoglobulins, are one of the main ways that the immune system fights disease. They are protein molecules that circulate in blood, and bind (mostly) to things that shouldn’t be there, like invading bacteria. These targets are called antigens. There are millions of different antibodies in the blood, with different protein sequences and different antigen specificities. The familiar Y shape of antibodies comes from the organization of their protein structure. Each molecule is made up of four protein chains: two identical heavy chains and two identical light chains, held together by disulfide bonds between the chains. Each chain is built up from two or more repeats of the immunoglobulin domain, a characteristic folded structure that’s used in many different proteins. Antibodies are produced and secreted into the blood by plasma cells, and each plasma cell makes only a single type of antibody.
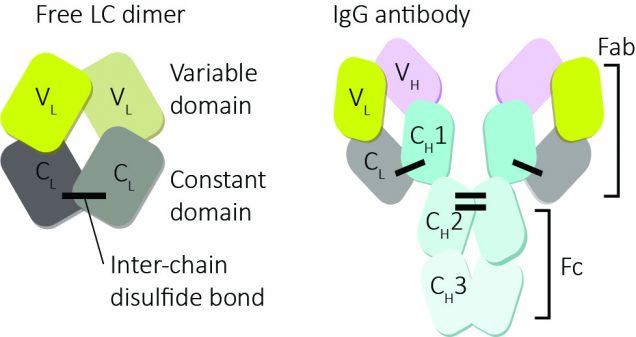
Heavy chains carry out most of an antibody’s function, but it’s the light chains that seem to cause AL amyloidosis. Light chains, as the name suggests, are smaller than heavy chains, and their sequences are less diverse, so they sometimes have a smaller role in antigen recognition than heavy chains. They also lack the effector domains, which heavy chains use for recruiting cells to whatever the antibody is bound to. Light chains are an important regulator of antibody production: heavy chains cannot be folded and exported without the light chains. This prevents them being released prematurely from cells, so that only mature, fully-built antibodies make it into the blood. The extra specificity provided by the light chains prevents heavy chains binding to the wrong things, which could cause the immune system to attack itself.
Plasma cells make slightly more light chains than heavy chains, and the excess light chains are released into the blood, either as monomers or dimers. They are then quickly removed from the blood by the kidneys, which work like a sieve to remove small proteins. Mature antibodies are too large to be filtered out, so they remain in the blood for much longer. A light chain molecule might circulate for a couple of hours before being filtered into the kidney and degraded by enzymes there. We don’t know of any ‘normal’ function for these free light chains, but because they’re abundant they serve as a useful marker for kidney function – too much light chain in your urine (proteinuria) suggests that there’s a problem.
If you’re unlucky enough to develop a plasma cell cancer such as multiple myeloma, then a single plasma cell will expand and multiply until it’s the dominant cell type in your bone marrow. This is bad news, but if it’s caught early it can be managed, although rarely cured. These cancerous cells have the same genotype, and secrete a single antibody, or sometimes just a single light chain. The presence of this single (“monoclonal”) protein in blood is diagnostic of a plasma cell cancer. Mostly, this protein is excreted via the kidneys in the same way as other light chains. But sometimes, the light chain molecules stick together and form deposits called amyloid fibrils. These fibrils cause organ damage where they accumulate, especially in the heart and kidneys. This is called amyloid antibody light chain amyloidosis (unintuitively abbreviated to AL amyloidosis) and affects one person in 100,000 each year. Treating the underlying cancer will cure the amyloid, but some patients – around 1000 per year in the USA – are too sick to tolerate chemotherapy. So an important goal is figuring out how to reduce the amyloid in patients’ blood and tissues without the toxic side effects of the cancer treatment.
Only some patients with plasma cell cancers develop amyloidosis. We don’t know why, but if we could work out the difference between patients with AL and those whose multiple myeloma presents without amyloid, we might find a way to treat the amyloidosis, and hence the cancer. One thing that seems to be important is the stability of the light chain protein. Light chain genes have unique DNA sequences in each plasma cell, the result of some complicated genetic rearrangements as the cells mature. These sequences result in different antigen specificities, but they also produce light chains with different stabilities. It seems that light chains which form amyloid are less stable than those that don’t, but we don’t know exactly how these differences in stability influence whether a particular protein will form amyloid.
AL amyloidosis is a rare disease, but it’s one of the more common ones. We call a number of conditions “rare diseases” because they’re unlikely to affect you or anyone you know, but there are an awful lot of them, and their combined burden is high. There’s been a huge change in the landscape of rare disease research and treatment over the last decade. Several factors have combined to brighten the prospects for treatment and management. Firstly, we’ve collectively made a lot of scientific progress. Probably the best examples of this are the RNA-directed therapies for rare diseases pioneered by Ionis and Alnylam. In addition, the FDA is more willing to approve drugs for “orphan” diseases that have no other treatment. Insurers and, to some extent, health services, are willing to pay high prices (Ionis and Alnylam again leading the way) for rare disease treatments because they can still represent good value compared to the existing standard of care. So the pharmaceutical industry has put more resources into what would have been considered impossibly niche areas a few years ago. And there are a lot of investors looking to fund high risk, high reward projects such as the newest shiny biotech company. It’s an exciting time to be working on rare diseases, because the path from basic research to an actual medicine is a little clearer and better trodden than it was.