Home
Description of Facility
The Boston University Cryogenic Electron Microscopy (BU CryoEM) Core Facility is a custom-designed microscope facility located at the Center for Advanced Biomedical Research on the BU Medical Campus. We are committed to providing exceptional instrumentation, training, and advice for researchers looking to answer their scientific questions using cryoEM, cryoET, and microED techniques. Our goal is to provide technical and scientific support for the entire cryoEM, cryoET, and microED workflow including sample preparation, data collection, and data processing.

What is CryoEM?
Cryogenic electron microscopy (cryoEM) is a versatile technique used to determine the structures of biological macromolecules and complexes at high resolutions. It has provided detailed insights into the organization and function of proteins, nucleic acids, and their complexes.
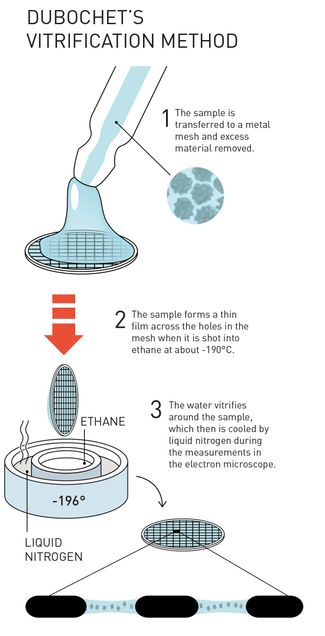
CryoEM involves freezing biological samples onto small, thin grids and rapidly cooling them in a process known as vitrification. Vitrification preserves the biological sample’s native structure with minimal damage. The process begins by depositing a small volume of purified sample onto cryoEM grids, blotting away excess liquid from the grid, then rapidly plunging it into liquid ethane cooled by liquid nitrogen. Vitrification prevents the formation of ice crystals and creates an amorphous ice layer that holds many copies of the sample for imaging. This amorphous ice layer is mostly transparent to the electron beam allowing for direct imaging of the sample.
Once the sample is frozen, it is transferred to an electron microscope, where it is imaged using with an electron beam. Unlike light microscopy, which uses photons to illuminate samples, electron microscopy employs electrons that have much shorter wavelengths, allowing for imaging of protein structure.
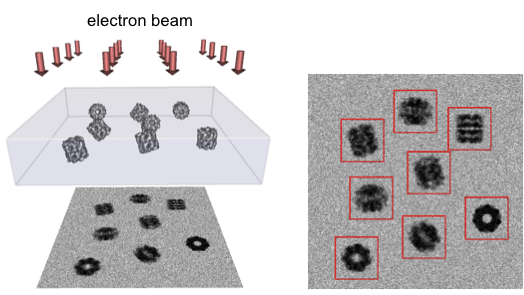
In a cryoEM data collection session, thousands of images are taken with each image containing hundreds of particles of sample frozen in random orientations. These images are then computationally processed to reconstruct a three-dimensional (3D) EM map of the sample, which can be used to create a detailed model of the sample.
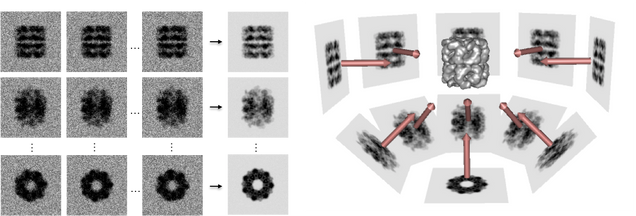
The reconstruction process involves aligning and averaging hundreds of thousands to millions of individual particle images of the sample to enhance signal-to-noise ratios and improve resolution. Sophisticated software and algorithms enable the determination of the sample’s 3D structure at near-atomic resolution.
CryoEM has several key advantages over X-ray crystallography as a method for structure determination. It does not require the sample to be crystallized, which can be a significant limitation for many biological macromolecules, especially larger complexes. In addition, cryoEM can produce multiple reconstructions from a single dataset showing the sample in multiple different conformations, revealing dynamic changes in its structure. This capability is particularly valuable for studying complex molecular machines and transient states that are difficult to capture.
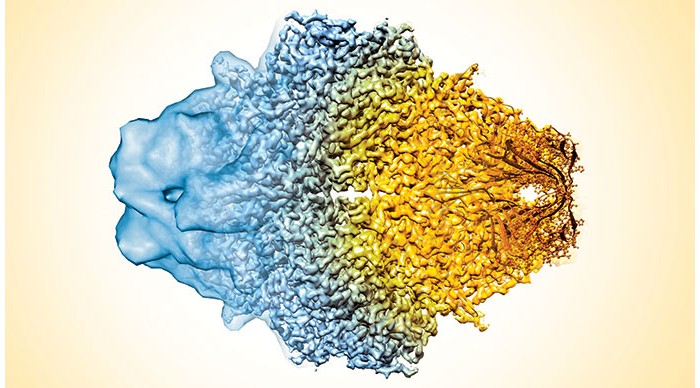
One of the most significant milestones in cryoEM was the development of direct electron detectors, which significantly improved the quality of images by reducing noise and increasing sensitivity. This advancement has allowed cryoEM to achieve resolutions previously thought to be unattainable, making it a powerful tool for structural biologists.
Recent applications of cryoEM have led to breakthroughs in understanding the structure and function of various biological systems. It has been instrumental in elucidating the structures of many important biological samples including the molecular machinery involved in cellular processes and virus particles. Notably, cryoEM determined the structure of the SARS-CoV-2 spike protein, which was crucial for vaccine development during the COVID-19 pandemic.
CryoEM is a transformative technology that enables researchers to visualize the structures of biological macromolecules at high resolutions. By leveraging advanced imaging and computational techniques, cryoEM provides detailed insights about the structures of biological macromolecules and their dynamics, expanding our understanding of basic biology and aiding in the development of new therapeutic treatments.
