Understanding the New Vaccines
Taylor Paiva
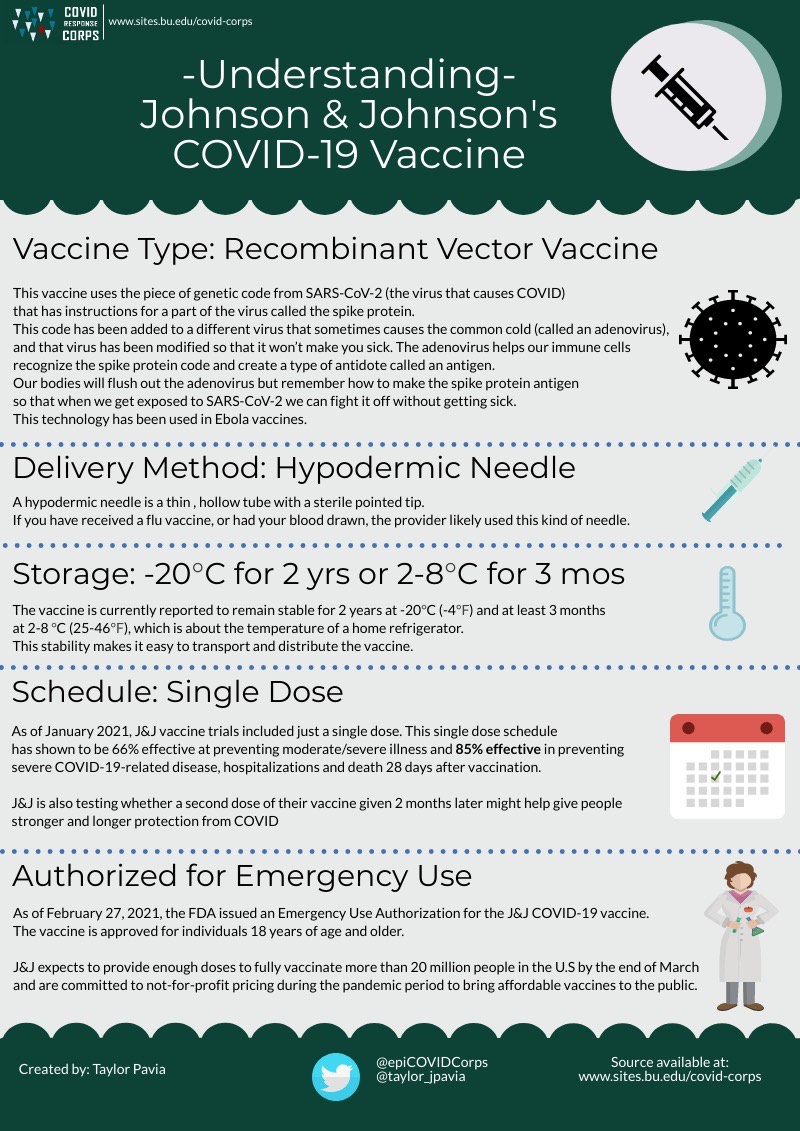
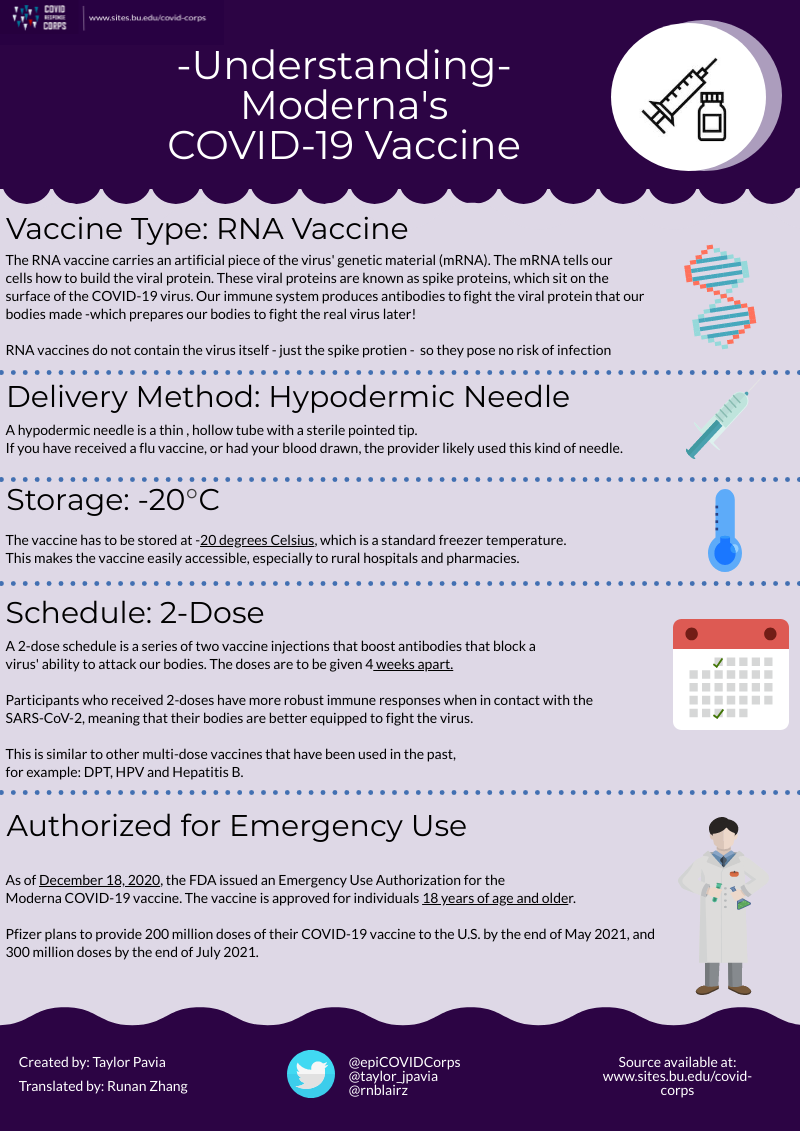
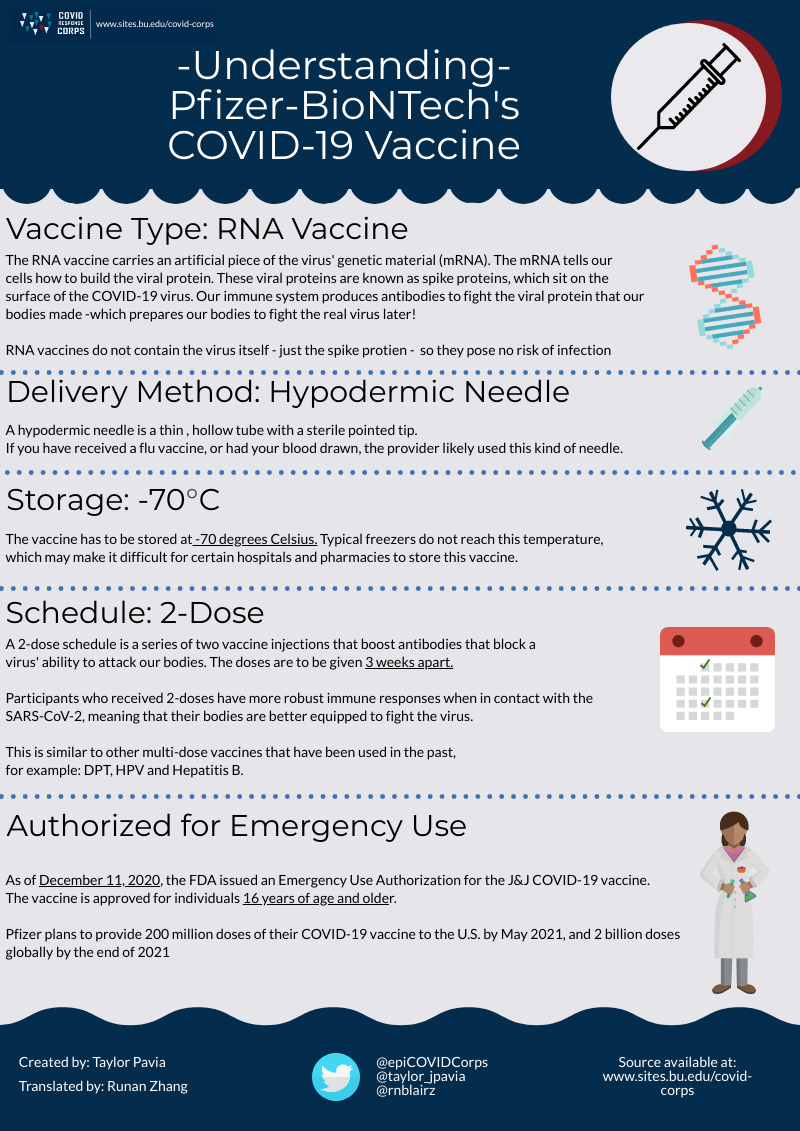
Three COVID-19 vaccines have been approved for emergency use as of March 2021: mRNA vaccines produced by Moderna and Pfizer/BioNTech, and an adenovirus vaccine produced by Johnson and Johnson. On November 9th, 2020, Pfizer announced that their vaccine candidate was found to be more than 90% effective in preventing COVID-19 infections. Just a few days later, on November 16th, Moderna announced that their vaccine candidate showed 95% efficacy. Both candidates here approved for Emergency Use Authorization (EUA) in December 2020. On February 27, 2021, Johnson and Johnson’s vaccine, which is 85% effective in preventing severe illness, was also authorized for emergency use.
Infographics
Moderna’s Vaccine
Pfizer’s Vaccine
Johnson and Johnson’s Vaccine