The Human Contraception Antibody (Video by Dr. Jai Marathe)
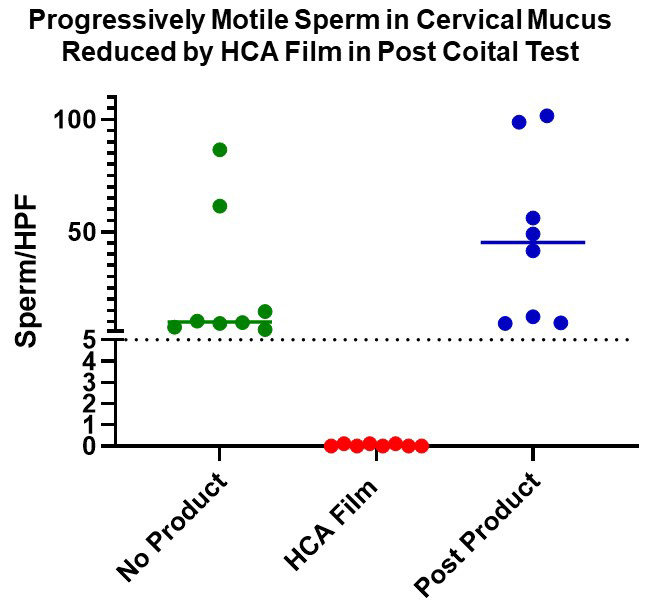
The Boston University Chobanian and Avedisian School of Medicine Contraception Development Research Center (CRC) is continuing the development of a new woman-controlled, on-demand contraception method based on the topical use of a potent antisperm monoclonal antibody (mAb), Human Contraception Antibody (HCA). HCA was originally identified as an antibody associated with infertility in humans and it rapidly agglutinates and immobilizes sperm [1]. It specifically reacts with a human male reproductive tract-restricted antigen, a unique glycoform of CD52 (CD52g) present on sperm and other cells in semen, but absent from cells and tissues not originating in the male reproductive tract [2]. Studies performed by the BU CRC to date: 1) produced GMP-grade HCA in Nicotiana, an innovative cost-effective mAb manufacturing platform [3]; 2) characterized the specificity and functional activity of the antibody in preclinical tests [2]; 3) incorporated HCA into a film (drug product, ZB-06) and tested it in IND-enabling studies; 4) obtained an exploratory IND from the FDA; and 5) recently completed and published a Phase 1 clinical trial with safety and efficacy (post-coital test, PCT) endpoints [25]. Results from the clinical trial indicate that ZB-06 is safe and highly effective in the PCT (mean number of progressively motile sperm per high powered field in cervical mucus: 25.9 in baseline cycle before ZB-06 use vs. 0.04 after ZB-06 use) (see figure below). Planning for ZB-06 Phase 2b/3 efficacy trials is currently in progress. HCA could be used alone for contraception, or in combination with mAbs or other microbicidal compounds against sexually transmitted pathogens in a Multipurpose Prevention Technology (MPT) product to enhance its impact, acceptability and marketability.
Progressively motile sperm (PMS) were significantly reduced 2-3 hours following intercourse with ZB-06 film [containing Human Contraception Antibody (HCA)] compared to intercourse with no product (p<0.0001) or post product visits (p<0.0001) (Thurman et al., 2023 [25]).
Contraception: The Unmet Need.
Of the 213 million pregnancies that occurred worldwide from 2015-19, over 40% were unintended [4]. In the United States, approximately 45% of pregnancies are unintended and forty percent of these pregnancies ended in abortion [5]. Numerous studies have indicated that unintended pregnancies are associated with an array of negative health, economic, social and psychological outcomes, particularly for women and children [6]. An estimated 225 million reproductive-aged women who want to avoid pregnancy are not using an effective contraceptive method [7]. Many women are averse to using highly effective hormonal contraceptive methods due to undesirable side effects, high cost and limited access to health providers. Barrier methods (male and female condoms, cervical cap, diaphragm) are widely available but are not discrete and are associated with a high failure rate due to imperfect use. The unintended pregnancy crisis is due in part to an unmet need for better access to modern effective contraception methods and new innovative methods that better address women’s needs [6]. Women would prefer a greater array of options for contraception. Easy to use, on-demand, non-hormonal, woman-controlled contraception may help fill the contraception gap. Currently two types of on-demand, non-barrier methods are approved for contraceptive use in the United States: topical spermicides containing Nonoxynol-9 and a topical vaginal pH regulating gel called Phexxi. However, fewer than 2% of women currently use these products in part due to their high failure rate and side effects. Both of these products have been reported to cause vaginal irritation [8,9], and N-9 has been associated with an increased risk of HIV acquisition [10]. The goal of our CRC is to develop a topical, on-demand, woman-controlled, non-hormonal method based on the use of an antisperm antibody called Human Contraception Antibody (HCA). We predict, based on preliminary data, that vaginal use of HCA film (ZB-06) will be more effective and have fewer side effects than the two currently available on-demand methods.
A Brief History of Antisperm Antibodies and Contraception
Antisperm antibodies commonly occur in infertility patients and are thought to cause infertility due to sperm agglutination and immobilization [11,12]. One antisperm antibody found in infertility patients is directed against a unique glycoform of CD52 (CD52g) that is present in abundant amounts on the surface of sperm. CD52g is produced and secreted by epithelial cells lining the lumen of the epididymis, vas deferens and seminal vesicles in the male genital tract [13]. It contains a short peptide sequence identical to CD52 found on lymphocytes, but a unique oligosaccharide sequence found only in the male reproductive tract (specifically not present on CD52 found on lymphocytes or elsewhere). CD52g also contains a glycosylphosphatidylinositol (GPI) anchor, that enables its incorporation into the plasma membrane of sperm as they mature in the epididymis [14]. The first anti-CD52g mAb, H6-3C4, was an IgM antibody produced in Japan from a hybridoma derived from B cells from an infertile woman [15]. Sperm antigens were investigated in the 1980’s by the World Health Organization for potential use as contraceptive vaccines (Dr. Anderson was a member of the WHO Birth Control Vaccines Task Force). The Herr laboratory produced a number of mouse antisperm mAbs, including MHS-8, another anti-CD52g mAb, and submitted them to a WHO-sponsored antisperm vaccine workshop. Out of over 100 antisperm monoclonal antibodies submitted to the workshop, the excellent profile of the MHS-8 mAb (workshop designation S19) pointed to CD52g as a promising antifertility vaccine candidate due to its unique expression in the male reproductive tract, potent antigenicity, and its potent sperm agglutination capability [16]. The WHO Birth Control Vaccines Task Force was discontinued in 1990, in part because of concern that the vaccines could have unseen side effects including permanent infertility.
Over the past 20 years the monoclonal antibody (mAb) field has matured and it is now possible to make reagent-grade monoclonal antibodies for clinical applications using new antibody production platforms [17]. This has opened up the possibility of using passive immunization for reversible contraception. Dr. Whaley and his associates founded a mAb production company, Mapp BioPharma in 2003, and a subsidiary company, ZabBio, focused on reproductive health in 2018. The companies use an innovative production platform, Nicotiana (tobacco plants), to make GMP-grade mAbs for clinical applications [18]. Drs. Anderson and Whaley have combined forces to make monoclonal antibodies for contraception and the prevention of sexually transmitted infections (STIs). In 2018, Anderson and Whaley received a CRC grant from NICHD and made an anti-CD52g IgG1 human mAb in Nicotiana. This antibody was named the Human Contraception Antibody (HCA). HCA was extensively tested in preclinical studies and was found to be specific for CD52g, immobilized sperm in fresh semen within seconds, and remained active under a variety of vaginal conditions. Furthermore, it did not cause vaginal irritation when added to vaginal tissue in vitro [2]. Mapp/ZabBio then manufactured a clinical-grade HCA film (ZB-06), obtained an exploratory IND, and the CRC team recently completed and published a Phase 1 clinical trial with safety and surrogate efficacy (postcoital test) endpoints [25]. The were no treatment emergent adverse events (TEAEs) determined to be related to the study product or study procedures, and ZB-06 showed excellent efficacy in the postcoital test: the mean number of progressively motile sperm per high powered field in cervical mucus in the baseline cycle before ZB-06 use was 25.9, vs. 0.04 after ZB-06 use. This is the first in-human clinical trial to use a topically applied monoclonal antibody for contraception.
Multipurpose Prevention Technology (MPT)
Sexually transmitted infections (STIs) are epidemic in the United States and worldwide and have far-reaching health, social and economic consequences. Each year more than 20 million men and women in the United States acquire an STI. The World Health Organization estimates the global annual incidence of curable STIs (bacterial STIs, syphilis and Trichomonas vaginalis) to be 357 million. The prevalence of viral STIs is estimated to exceed 800 million. Over 2.5 million new HIV-1 infections are acquired annually, most through sexual intercourse. Some STIs adversely affect fertility and cause severe pathology in newborns. Others such as HIV-1 and high-risk human papillomavirus (HPV) strains can cause severe morbidity often leading to death. Thus far, only a few vaccines have been developed to prevent STIs (i.e., HPV and Hepatitis vaccines). Despite the expenditure of billions of dollars over the past 30 years in HIV research, vaccine and topical microbicide strategies have been ineffective or only marginally effective in preventing HIV sexual transmission in clinical trials. In surveys, women have overwhelmingly expressed interest in MPT products that protect against both STIs and unplanned pregnancies. Several MPT products are under development including combinations of hormonal contraception and antiretroviral drugs. In 2013, Drs. Anderson and Whaley received a grant from NIAID to make mAbs against HIV and HSV-2 in Nicotiana to use topically for STI protection; these antibodies were tested in preclinical and clinical studies with promising results [19]. Our group is the first to suggest the use of combinations of monoclonal antibodies representing different contraception and STI targets for MPT [20]. Although it is beyond the scope of this CRC project which focuses solely on the contraceptive antibody, it is our plan to use a combination of anti-STI and contraceptive antibodies in future studies to further evaluate antibody-based MPT safety and efficacy.
mRNA-Mediated Topical Delivery System for HCA
The CRC team at BU is collaborating with Emory University and the University of Louisiana at Lafayette on an exciting project to develop an mRNA-mediated topical delivery system for HCA. Delivery is achieved via exposure of the female reproductive tract to aerosolized mRNAs encoding HCA. This method allows for cost-effective, rapid expression and controllable retention of HCA within the vaginal epithelium for contraceptive protection. We are currently working to demonstrate pre-clinical feasibility in animal models. Our long-term goal is to combine mRNAs for co-expression of HCA and anti-STI antibodies as an MPT.
References
- Isojima S, Tsuchiya K, Koyama K, Tanaka C, Naka O, Adachi H. Further studies on sperm-immobilizing antibody found in sera of unexplained cases of sterility in women. Am J Obstet Gynecol. 1972;112(2):199-207.
- Baldeon-Vaca G, Marathe JG, Politch JA, et al. Production and characterization of a human antisperm monoclonal antibody against CD52g for topical contraception in women. EBioMedicine. 2021;69:103478.
- Anderson DJ, Politch JA, Cone RA, et al. Engineering monoclonal antibody-based contraception and multipurpose prevention technologies. Biol Reprod. 2020;103(2):275-285.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8(9):e1152-e1161.
- Finer LB, Zolna MR. Declines in Unintended Pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843-852.
- Anderson DJ. Population and the Environment – Time for Another Contraception Revolution. N Engl J Med. 2019;381(5):397-399.
- Sedgh G, Hussain R. Reasons for contraceptive nonuse among women having unmet need for contraception in developing countries. Stud Fam Plann. 2014;45(2):151-169.
- Fichorova RN, Tucker LD, Anderson DJ. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;184(4):418-428.
- Thomas MA, Chappell BT, Maximos B, Culwell KR, Dart C, Howard B. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360(9338):971-977.
- Bronson RA. Antisperm antibodies: a critical evaluation and clinical guidelines. J Reprod Immunol. 1999;45(2):159-183.
- Ustay K, Behrman SJ, Tanapongpipatana S. Clinical application of antisperm-antibody test. Univ Mich Med Cent J. 1967;33(5):225-227.
- Norton EJ, Diekman AB, Westbrook VA, et al. A male genital tract-specific carbohydrate epitope on human CD52: implications for immunocontraception. Tissue Antigens. 2002;60(5):354-364.
- Diekman AB, Norton EJ, Klotz KL, et al. N-linked glycan of a sperm CD52 glycoform associated with human infertility. FASEB J. 1999;13(11):1303-1313.
- Isojima S, Kameda K, Tsuji Y, Shigeta M, Ikeda Y, Koyama K. Establishment and characterization of a human hybridoma secreting monoclonal antibody with high titers of sperm immobilizing and agglutinating activities against human seminal plasma. J Reprod Immunol. 1987;10(1):67-78.
- Anderson DJ, Johnson PM, Alexander NJ, Jones WR, Griffin PD. Monoclonal antibodies to human trophoblast and sperm antigens: report of two WHO-sponsored workshops, June 30, 1986–Toronto, Canada. J Reprod Immunol. 1987;10(3):231-257.
- Anderson DJ, Politch JA, Kadasia K, Baldeon G, Villinger F, Whaley KJ. Systemic and topical use of monoclonal antibodies to prevent the sexual transmission of HIV. AIDS. 2017;31(11):1505-1517.
- Whaley KJ, Hiatt A, Zeitlin L. Emerging antibody products and Nicotiana manufacturing. Hum Vaccin. 2011;7(3):349-356.
- Politch JA, Cu-Uvin S, Moench TR, et al. Safety, acceptability, and pharmacokinetics of a monoclonal antibody-based vaginal multipurpose prevention film (MB66): A Phase I randomized trial. PLoS Med. 2021;18(2):e1003495.
- Whaley KJ, Zeitlin L. Antibody-based concepts for multipurpose prevention technologies. Antiviral Res. 2013;100 Suppl:S48-53.
- Castle PE, Karp DA, Zeitlin L, et al. Human monoclonal antibody stability and activity at vaginal pH. J Reprod Immunol. 2002;56(1-2):61-76.
- Castle PE, Whaley KJ, Hoen TE, Moench TR, Cone RA. Contraceptive effect of sperm-agglutinating monoclonal antibodies in rabbits. Biol Reprod. 1997;56(1):153-159.
- Cone RA, Hoen T, Wong X, Abusuwwa R, Anderson DJ, Moench TR. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect Dis. 2006;6:90.
- Zhao C, Gunawardana M, Villinger F, et al. Pharmacokinetics and preliminary safety of pod-intravaginal rings delivering the monoclonal antibody VRC01-N for HIV prophylaxis in a macaque model. Antimicrob Agents Chemother. 2017;61(7):e02465-02416.
- Thurman AR, Moench TR, Hoke M, et al. ZB-06, a vaginal film containing an engineered human contraceptive antibody (HC4-N), demonstrates safety and efficacy in a phase 1 postcoital test and safety study. Am J Obstet Gynecol. 2023 Mar 2;S0002-9378(23)00139-4. doi: 10.1016/j.ajog.2023.02.024. Online ahead of print.
- Mausser E, Nador E, Politch JA, et al. LALAPG variant of the Human Contraception Antibody (HCA) reduces Fc-mediated effector functions while maintaining sperm agglutination activity. PLoS One. 2023 Mar 30;18(3):e0282147. doi: 10.1371/journal.pone.0282147. eCollection 2023.