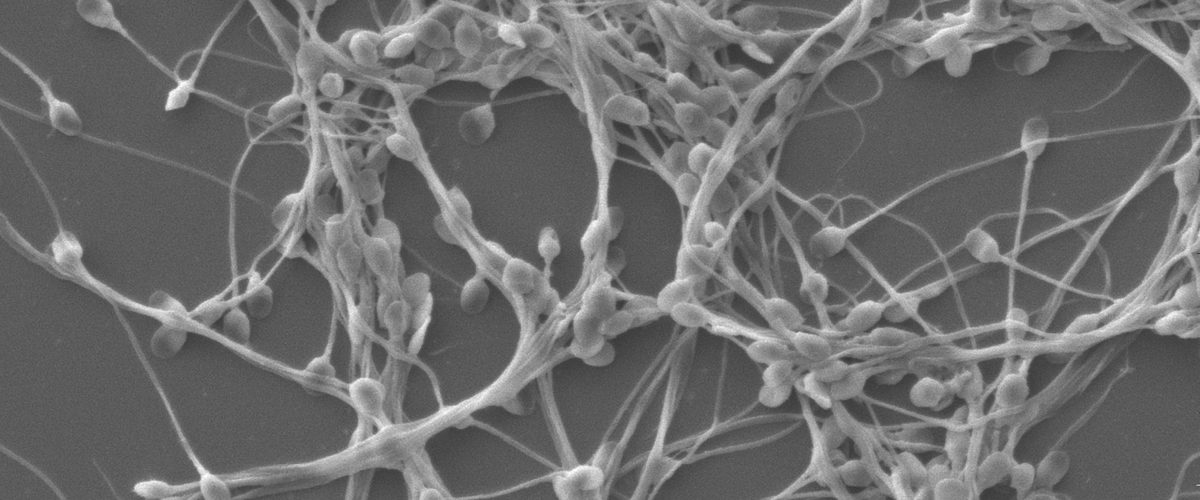
Scanning electron micrograph of human sperm agglutinated by HCA (Drs. Jai Marathe and Jeffrey Pudney)
 Kevin J. Whaley, Ph.D., Deborah J. Anderson, Ph.D. and Thomas R. Moench, M.D.
Kevin J. Whaley, Ph.D., Deborah J. Anderson, Ph.D. and Thomas R. Moench, M.D.
Overview
In 1994, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) was directed by Congress to establish three research centers for the purpose of improving methods of contraception (42 USC 285g-5). Since 2018, the Boston University Chobanian and Avedisian School of Medicine has been the recipient of one the national contraception research center grants, co-directed by Prof. Deborah Anderson, Dept. of Medicine/Infectious Diseases and Dr. Kevin Whaley, CEO of ZabBio (San Diego CA).
The overarching goal of the BU Contraception Research Center is to develop innovative monoclonal antibody (mAb)-based contraceptive and multipurpose prevention technology (MPT) products with the potential to improve the lives of millions of women and men globally by addressing two concurrent reproductive health crises: an unacceptably high rate of unintended pregnancies in both developing and developed countries, and an epidemic of sexually transmitted infections (STIs). With funding from NIAID we developed a vaginal film, MB66, containing mAbs against HIV-1 and HSV-2 [the first components of our MPT product]. MB66 was safe and showed good ex vivo efficacy in a Phase 1 Clinical Trial. Under our current CRC program, we engineered, produced and characterized a GMP-grade human antisperm mAb “Human Contraception Antibody (HCA)”, and manufactured ZB-06, a vaginal film containing HCA for topical vaginal application. ZB-06 was tested in a Phase 1 Clinical Trial in women with safety and exploratory efficacy (postcoital test) endpoints. The published results of this trial indicate that ZB-06 is safe and highly effective at preventing motile sperm from entering the endocervix, a key correlate of contraceptive efficacy.
We are now planning to advance ZB-06 through additional clinical trials. ZabBio is currently improving upon and scaling up the production of ZB-06 for use in IND-enabling studies and in Phase 1 and Phase 2a clinical trials. ZabBio will file all paperwork required by the FDA to obtain an IND for the clinical trials, maintain the IND and monitor the clinical trials. The two clinical trials, to be conducted at Eastern Virginia Medical School and the University of Texas Health Science Center at San Antonio (UT Health), will be: 1) a Phase 1 safety and PK study of single dose vs. multiple dose application of ZB-06, and 2) a Phase 2a dose-finding study of ZB-06 film with safety and postcoital test efficacy endpoints. Another project in this center grant, based at Boston University School of Medicine (BUSM) and Magee-Women’s Research Institute, will improve upon the vaginal film design and conduct essential safety studies to support the clinical trials. An Administrative Core, also at BUSM, will provide leadership and fiscal management for all projects and database and statistical support for the clinical trials. By the end of the project we expect that ZB-06 will be positioned for Phase 2b contraceptive efficacy testing by the NIH Contraceptive Trial Network.