Research
Accumulating evidence suggests key roles of dysbalanced innate immune responses, inflammation and host-pathogen interactions during pneumonia, ARDS, sepsis and inflammatory bowel disease (IBD). The presence of pathogen-associated molecular patterns (PAMPs) provokes the release of a plethora of inflammatory mediators (e.g. cytokines, complement anaphylatoxins) accompanied by complex interactions of intracellular signaling cascades. The focus of our experimental research projects is on the cellular and molecular mechanisms of these innate immune responses including macrophage functions, neutrophil extracellular trap (NETs) formation, cytokines/chemokines, complement, signaling pathways and novel bacterial PAMPs (e.g. polyphosphates). Our research employs a diverse array of cutting-edge experimental models and methodologies including transgenic mouse models, next-generation sequencing technologies bulk RNA-seq and CITE-seq, proteomic analyses via LC-MS, immunoassays including ELISA and Luminex, molecular and cellular techniques such as Western blotting, flow cytometry, RT-qPCR, and comprehensive bioinformatics analyses to deepen our understanding of underlying mechanisms.
Role of complement C5a-C5AR1/C5AR2 signaling in acute lung injury
The complement system serves as a critical component of innate immunity and plays a significant role in modulating infection-associated inflammation. Acute respiratory distress syndrome (ARDS), often triggered by severe bacterial pneumonia, represents a serious clinical challenge characterized by overwhelming inflammation and tissue damage. Activation of the complement cascade through the generation of the potent complement anaphylatoxin C5a has been implicated in driving this excessive immune response. C5a interacts with its homologous G-protein coupled rec eptors, C5aR1 and C5aR2, encoded by two adjacent genes. The functional roles of C5aR1 and C5aR2 across different tissues remain incompletely understood, with some controversy surrounding the role of C5aR2. To better dissect these roles, we have generated a genetically engineered mouse strain with a ~12.6 kb deletion of both C5aRs (C5aR1/2-/-) by CRISPR/Cas9 gene editing, and another mouse strain for reporting and conditional deletion of C5aR2 (C5aR2LacZ; C5aR2flox). Our ongoing research explores the combined roles of C5aR1 and C5aR2 in host defense and inflammation during bacterial pneumonia caused by Streptococcus pneumoniae, a leading cause of pneumonia-related death worldwide.
Molecular mechanisms regulating myeloid responses in infection and tissue injury
A key research interest of our lab is investigating the immunological signaling pathways that (dys)regulate myeloid cell responses during infection and tissue injury. Our ongoing studies explore how these pathways affect critical processes including pathogen clearance, inflammatory signaling, immune cell activation, and tissue homeostasis. By integrating genetic and cellular models, we aim to decipher how perturbations in these signaling networks contribute to both protective immunity and disease severity. This work encompasses different molecules including the ion channel protein PACC1 involved in phagolysosomal functioning, the macrophage-derived immunomodulatory cytokine IL-27 and its receptor IL-27RA that shape lymphocyte activity, and the cytosolic RNA sensing receptor complex MAVS/MDA5/RIGI. Together, these studies aim to deepen our understanding of the molecular events that modulate myeloid immune responses and the balance between pathogen clearance and tissue damage in bacterial pneumonia and sepsis.
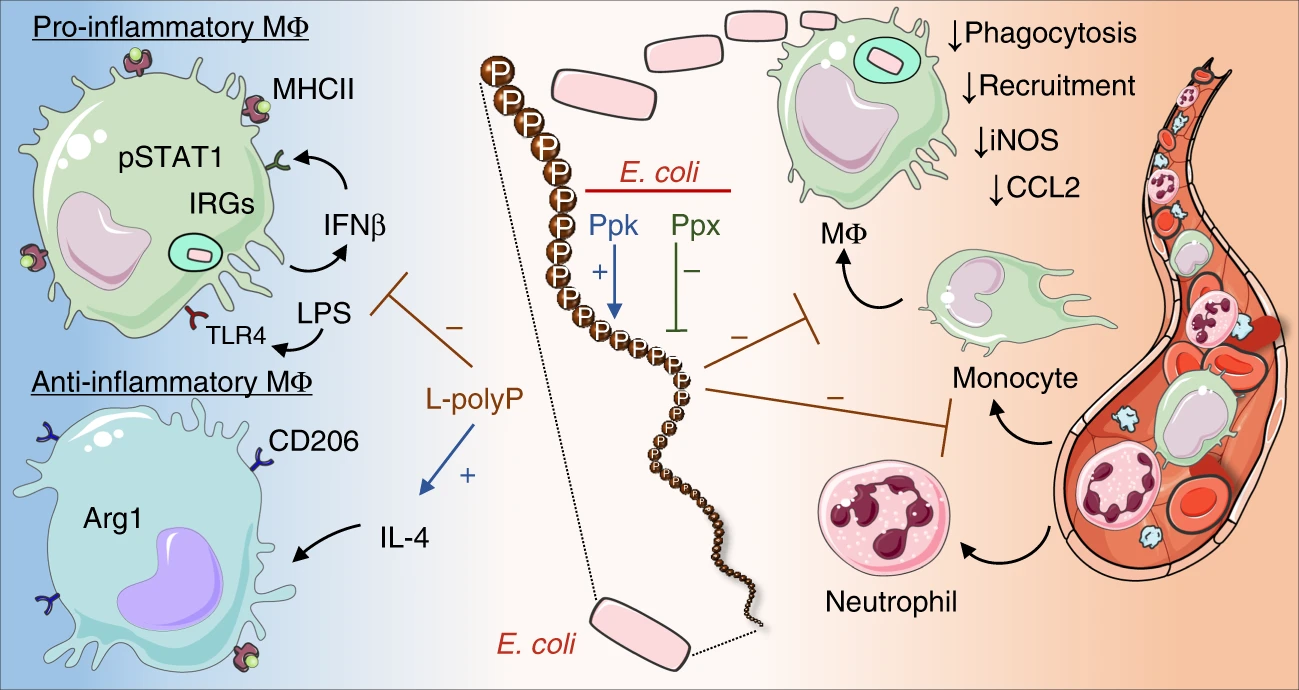
Role of bacterial polyphosphates in maladaptive inflammation
Polyphosphates are linear polymers of inorganic phosphate (Pi) residues that are present in all living organisms. Bacterial metabolism accumulates long-chains of polyphosphates (Pi: n≥700) whereas mammalian cells typically synthesize short-chain polyphosphates (Pi: n<100). Our research has revealed that these bacterial long-chain polyphosphates disrupt protective host immune responses and worsen outcomes in septic peritonitis after cecum ligation and puncture (Roewe et al. Nat Comm. 2020 PubMed). Many bacterial pathogens, including Escherichia coli and Legionella spp., possess enzymes such as polyphosphate kinase and exopolyphosphatases, which regulate polyphosphate metabolism and accumulation. Our lab is actively investigating how these bacterial long-chain polyphosphates interact with and modulate host mucosal immunity, potentially driving immune dysregulation in both acute infections (Legionnaires’ disease), and chronic inflammatory disorders (inflammatory bowel disease).