Our Newest Publications in Energy Conversion, Storage, and Generation
| 1. Morey, M., G. Nagaro, A. Halder, S. Sharifzadeh, E. Ryan, A framework for nucleation in electrochemical systems and the effect of surface energy on dendrite growth, Journal of Energy Storage, 2024, 92, 112144. |
2. A.B. Resing, C. Fukuda, J.G. Werner. (2023) Architected Low-Tortuosity Electrodes with Tunable Porosity from Nonequilibrium Soft-Matter Processing, Advanced Materials. |
Electrode-Electrolyte Interfacial Modeling – Dendrite Growth in Lithium Batteries (Emily Ryan)
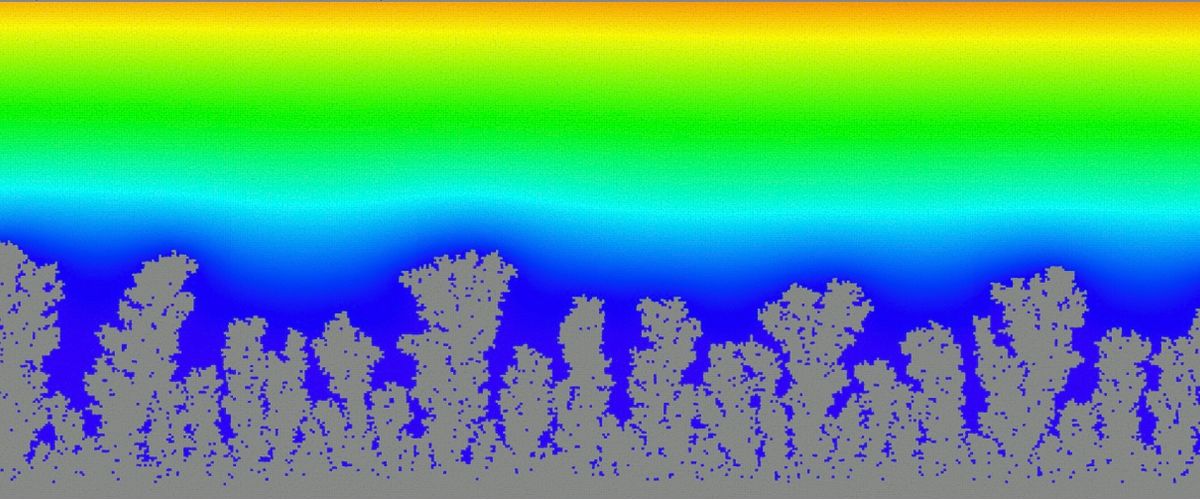
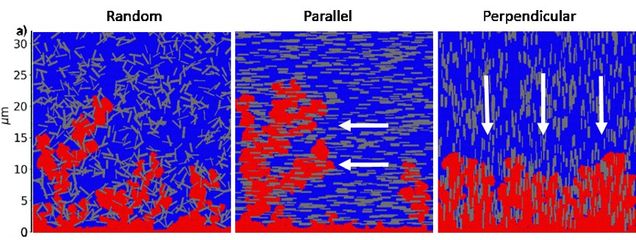 Prof. Ryan’s group uses meso-scale computational fluid dynamics modeling to understand the chemical-physical processes during charge and discharge in lithium metal batteries that lead to dendrite growth. Dendrite growth causes a decrease in performance due to lost lithium, and can cause thermal runaway and battery failure. Modeling aims to understand the critical physics driving dendrite growth and develop strategies to suppress growth.
Prof. Ryan’s group uses meso-scale computational fluid dynamics modeling to understand the chemical-physical processes during charge and discharge in lithium metal batteries that lead to dendrite growth. Dendrite growth causes a decrease in performance due to lost lithium, and can cause thermal runaway and battery failure. Modeling aims to understand the critical physics driving dendrite growth and develop strategies to suppress growth.
Reversible Solid Oxide Electrochemical Cells for Energy Storage (Srikanth Gopalan)
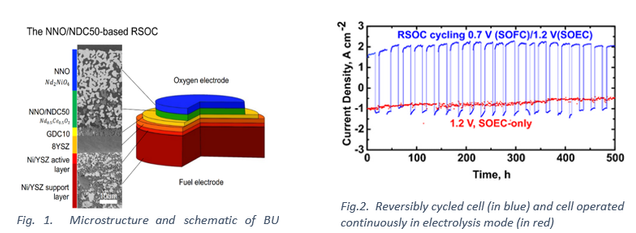
With increasing amounts of solar and wind being incorporated into our energy portfolio, the intermittency of these sources over shorter and longer duration remains a central challenge. We are exploring reversible solid oxide cells (rSOCs) to convert renewable electricity when available into hydrogen, and the hydrogen back to electricity when needed. Success with such an approach requires highly reversible oxygen and fuel electrodes under continuous cycling between the electrolysis and fuel cell modes of operation. In this project, we are exploring Ruddlesden-Popper phases with oxygen hyperstoichiometry (interstitials) as reversible oxygen electrodes, and modified (infiltrated) Ni-yttria stabilized zirconia (Ni-YSZ) cermet electrodes as reversible fuel electrodes. Such cells have proven to be highly reversible and cyclable between fuel cell and electrolysis modes of operation. An interesting research outcome in this project is that the performance degradation of the cell when cycled between operating modes is significantly lower than when operated as electrolysis cells.
Using Machine Learning to Diagnose Failure in Lithium Ion Batteries (Emily Ryan)
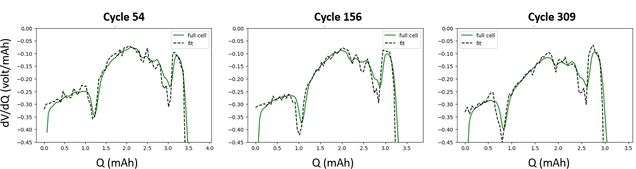
In collaboration with Argonne National Laboratory, Prof. Ryan’s group is using machine learning to facilitate battery diagnostics to reduce analysis time and elucidate failure mechanisms.
Comparison of chromium poisoning between lanthanum strontium manganite and lanthanum strontium ferrite composite cathodes in solid oxide fuel cells (Uday Pal, Srikanth Gopalan, Soumendra Basu)
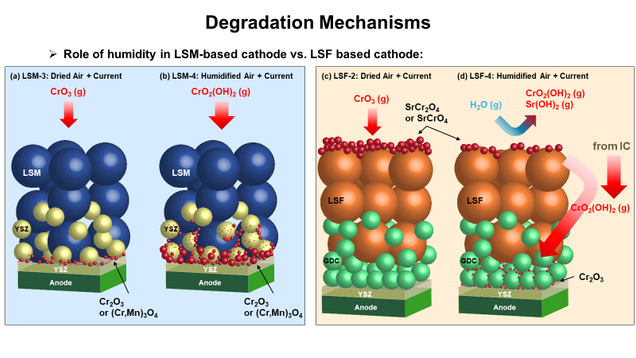
Degradation mechanisms of chromium poisoning have been extensively investigated for last two decades on various cathode materials for solid oxide fuel cells. While most researchers used half-cell experiments to investigate the effects of chromium impurities, Profs. Pal, Gopalan, and Basu are comparing the degradation phenomena of LSM-YSZ and LSF-GDC cathodes in full cells under different operating conditions, namely polarized current density and humidity over the cathode.
Under cathodic load, performance degradation of LSF-GDC cathode is significantly less than that of LSM-YSZ cathode. Most interestingly, under humidified air condition, LSF-based cell hardly experiences performance degradation while LSM-based cell shows a catastrophic degradation. Cr deposition in LSM-based cells occurs mainly near the electrolyte under both dry and humidified air condition. In contrast, Cr deposition in LSF-based cell concentrates at the cathode surface under dry air condition, but concentrates both at cathode surface and near electrolyte under humidified air condition. Microstructure analysis reveals Mn-associated Cr deposits in LSM-based cathode, and Sr- and Fe- associated Cr deposits in LSF-based cathode.
With the help of distribution of relaxation time analysis, the different electrochemical behaviors of LSM- and LSF-based cells are carefully interpreted. The effects of electrochemical deposition, Sr surface segregation, and humidity on LSM- and LSF-based cathodes are discussed.