ADEPTT
ADEPTT:
A summary of the Uganda ARCH cohort renewal study (2016-2021)
The Uganda ARCH team is launching their renewal study, which builds on past research of alcohol use and HIV in Mbarara, Uganda. This new study will examine a common comorbidity among HIV-infected individuals – tuberculosis (TB). TB is the leading cause of mortality in persons with HIV globally; however, treatment is often not provided for individuals who drink heavily due to concerns of increased hepatotoxicity if not regularly monitored. The Alcohol Drinkers’ Exposure to Preventive Therapy for TB (ADEPTT) study will examine the safety and tolerability of TB preventive therapy with isoniazid (INH) for HIV-infected drinkers in Mbarara, Uganda. Toxicity for drinkers has not been well-studied despite evidence that heavy drinking increases the risk factor for active TB and that INH has been proven to reduce mortality and TB in HIV-infected individuals. This single-arm treatment study of 300 (200 drinkers and 100 abstainers) allows for examination of the safety and tolerability of TB preventive therapy, which will help determine if the benefits outweigh the risks. The specific aims of ADEPTT are:
- To examine the safety and tolerability of TB preventive therapy for HIV-infected drinkers, measured by hepatotoxicity and treatment discontinuation rates, overall and by level of drinking.
- To estimate the level of TB preventive therapy adherence, overall and by level of drinking.
- To determine whether the benefits of providing TB preventive therapy to HIV-infected drinkers in resource limited settings outweigh the risks compared to no treatment.
The ADEPTT team hypothesizes that 1) adherence will be greater among those with the lowest drinking levels; 2) providing TB preventive therapy will result in longer life expectancy and quality-adjusted life expectancy than not providing TB preventive therapy (current standard of care). The design and flow of the study are also as follows:
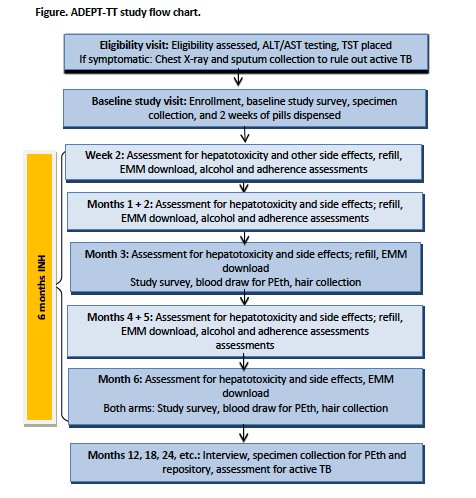
ADEPTT will recruit 300 participants, 100 of which will be abstainers and 200 will be current drinkers, who are HIV-infected, latent TB infected, and on ART. Data will be collected at monthly visits for hepatotoxicity and other side effects during the first six months, and bi-annually for the remainder of the study. Objective measures of adherence will be measured through electronic medication monitoring that records pill bottle opening and INH concentration in hair samples.
Study recruitment began in April 2017. ADEPTT has the potential to provide critical data for guidelines for TB preventive therapy in drinkers with HIV to reduce morbidity and mortality.
Want to learn more about ADEPTT? Click here for our April – June 2017 newsletter issue spotlight on Karen Jacobson.
 l-r: Jeffrey Samet, Christine Ngabirano, Judy Hahn, Nneka Emenyonu, and Robin Fatch
l-r: Jeffrey Samet, Christine Ngabirano, Judy Hahn, Nneka Emenyonu, and Robin Fatch
- Principal Investigator:
- Judith Hahn, PhD, MA, Associate Professor in Residence, Infectious Disease at San Francisco General Hospital, University of California, San Francisco
- Co-investigators:
- Monica Gandhi, MD, MPH, Professor of Medicine and Associate Division Chief of the Division of HIV, Infectious Diseases, and Global Medicine, at the University of San Francisco School of Medicine
- Karen Jacobson, MD, MPH, Assistant Professor of Medicine at Boston University School of Medicine
- Benjamin Linas, MD, MPH, Assistant Professor of Medicine and Director of HIV Epidemiology and Outcomes Research Unit at Boston University School of Medicine
- Winnie Muyindike, MBChB, MMED, Director of ISS Clinic, at the Mbarara University of Science and Technology (MUST)
- Debbie Cheng, ScD, Professor of Biostatistics, Boston University School of Public Health
- Study Biostatistician:
- Robin Fatch, MPH, Statistician/Data Manager, Infectious Diseases, Department of Medicine at the University of San Francisco
- Study Contact:
- Nneka Emenyonu, DrPH, MPH, Project Director at University of California, San Francisco, Emenyonu@ucsf.edu