Tagged: Srijesa Khasnabish
The Paradox of Cell Death
“Apoptosis” is Greek word that literally means “falling of the petals from a flower or leaves from a tree” [1]. This description contrasts with the typical textbook definition: apoptosis is programmed cell death, caused by the Diablo gene. The literal translation stresses the evolutionary significance and benefit of apoptosis. While apoptosis can be detrimental, it is also important to understand the reason why nature would select for such a seemingly negative process that in fact has its benefits.
Apoptosis is important for maintaining a balance between cell proliferation and cell death. Families of proteases called caspases are responsible for cell death. Initiator caspases activate other executioner caspases, which continue to activate other executioner caspases. The Bcl2 family of proteins can either activate or deactivate caspases. Specifically Bax and Bak facilitate cell death because they cause cytochrome C to be released, leading to the assembly of the apoptosome. Another process for cell death is called necrosis – when a plasma membrane ruptures thus spreading the contents of the dying cells. This is different from apoptosis because typically phagocytes recognize cells undergoing apoptosis and engulf the cells and its contents, thus preventing them damage to other surrounding cells [2].
A protein relevant to apoptosis is the transcription regulator protein p53, which codes for a CDK inhibitor protein called p21 (a Cdk inhibitor protein). In response to DNA damage in the G1 phase of the cell cycle, p53 levels increase and the generated p21 binds to the G1/S-CDK to stop the cell from reaching the S phase. In cases where DNA damage cannot be repaired, p53 causes apoptosis because it is better for the cell cycle not to proceed with the damaged DNA. If p53 is nonfunctional, this has serious consequences for the cell because its leads to the replication of damaged DNA, leading to mutation and the generation of cancerous cells [2]. I find it astonishing that an issue with the expression of one protein, in this case p53, can lead to development of life-threatening diseases like cancer.
Furthermore, apoptosis plays an interesting role in Alzheimer’s disease (AD). AD is caused by AB plaques, which are aggregates of a misfolded version of the amyloid precursor protein (APP). AB is generated when APP is cleaved by B-secretase (or BACE) in the amyloidogenic pathway. Current research shows that the death of neuronal and glial cells in the cerebral cortex and hippocampus is related to the cognitive decline that is characteristic of AD [3]. Only part of this neuronal cell death is caused by apoptosis, meaning the rest is mediated by necrosis and other different forms of apoptosis. Research implies that Bak and Bad rather than Bax (a part of the Bcl2 family) [3], therefore a potential AD treatment could involve a method for inhibiting the expression of Bak/Bad.
Cancer can be caused by a lack of apoptosis in harmful cells, thus allowing them to proliferate and cause tumors. Interestingly, the p53 gene is found in half of all human cancers [2]. Typically in tumors scientists have observed that the main regulators of the cell cycle are altered, thus impacting aspects of proliferative control such as cell cycle checkpoints and how the cell responds to DNA damage. Therefore, a possible cancer treatment could involve finding a way to induce tumor-selective cell death. However, a challenge with this approach is finding a way to do so without harming normal cell function or normal cell homeostasis [4]. If scientists could discover a molecule specific to tumor cells or molecules that are regulated differently in tumor cells than in normal cells, this approach could be potentially beneficial.
~ Srijesa Khasnabish
Sources
[1] Zhivotovsky, B (2002) From the Nematode and Mammals Back to the Pine Tree: On the asdfasdfDiversity and Evolution of Programmed Cell Death Cell Death and Differentiation 9, asdfasdf867-869 doi:10.1038/sj.cdd.4401084
[2] Alberts, Bruce (2014) Essential Cell Biology. New York: Garland Science
[3] Shimohama, S (2000) Apoptosis in Alzheimer’s Disease – An Update Apoptosis 5, 9-16
[4] Kasibhatla S, Tseng B (2003) Why Target Apoptosis in Cancer Treatment? Molecular asdfasdfCancer Therapeutics 2, 573
The Mystery of the Human Connectome
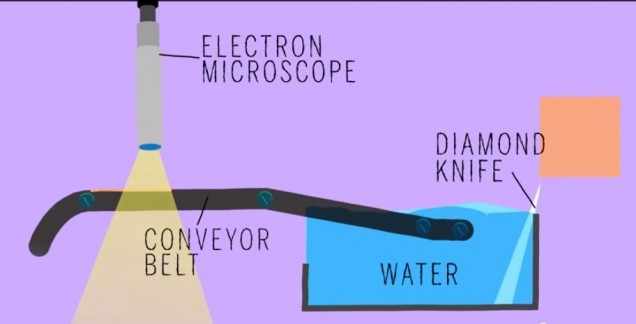
Did anyone read that article in BU today last week called “Untangling the Connectome?” In case you didn’t, here’s a brief synopsis. Dr. Kasthuri and his lab members are working on identifying the connections between the neurons in the human brain. This has been done before for C. elegans, which have only 302 neurons (compared to our 100 trillion). So, imagine how complex this project is and how much data is contained in one tiny slice of human brain?! In the article, Kasthuri said that a brain slice with the volume of “a millimeter cubed, at the resolution we would like, is about two million gigabytes of data” [1].That’s crazy. The Kasthuri lab implemented a clever method (Figure 1) to take images of 30 nm brain slices that provide the high resolution pictures they’re looking for.
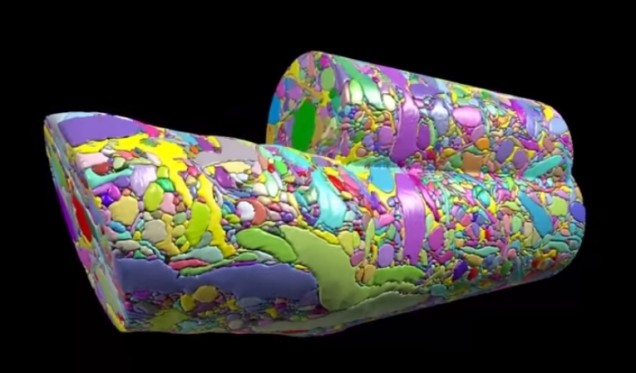
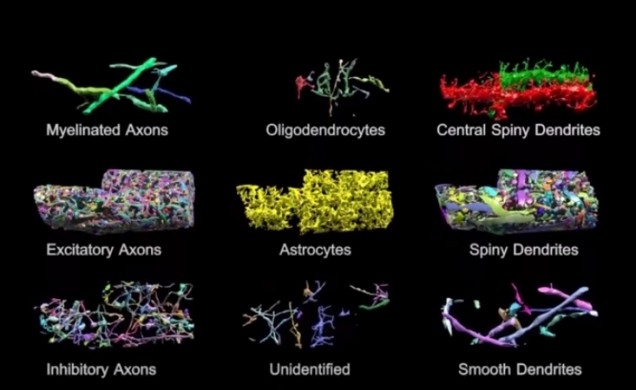
Basically, the brain volume is immersed in plastic and cut with a diamond knife to ensure precision. Since the slices are somewhat floppy, they are collected in water then picked up by a conveyor belt. While on the conveyor belt, an electron microscope takes a quick shot of the brain tissue and next a collection of images is put together to create a accurate image of the initial brain volume. Methods can be used to break down the image of the brain volume into components such as axons, dendrites, etc. (Figure 2). Pretty cool, right? The implications of this research is that understanding the connections in our brain can teach us about brain development and memory functions.
The Human Connectome project at Mass General Hospital (MGH) is working on developing high resolution neuroimaging methods. They’re working on figuring out how varying white matter fiber connectivity correlates to brain function and how cytoarchitectonics (the pattern of neuron organization/density in different areas of the brain, used to decipher Brodmann’s areas in the brain) can influence brain connectivity [2]. Methods they’re using include diffusion spectrum imaging (DSI), which is similar to diffusion tensor imaging (DTI) as both allow scientists to observe white matter connectivity since water diffuses parallel to white matter tracts. DTI measures the preferred and mean direction of the diffusion of water while DSI measures it in many different directions [3]. Scientists at MGH are also working on developing tools for high angular diffusion imaging (HARDI), which “address[es] the challenge of imaging crossing fibers by applying a stronger magnetic gradient for a longer time and capturing many more images of diffusion in various directions” [3].
Researchers are hoping to find patterns of connectivity by deciphering how our 100 trillion neurons are linked. This information could help scientists better understand the roots of different neurological or neurodegenerative diseases where perhaps neural connectivity differs significantly. This is a challenging project but the results of it could be a major breakthrough for neuroscience.
~ Srijesa K.
Sources:
[1] "Untangling the Connectome"
[3] "White's the Matter: A basic guide to white matter imaging using diffusion MRI"
Optogenetics: Using Light to Turn Memories On and Off

Scientists have known about rhodopsins that are responsible for sensing light for a while. What if there was a way to insert those rhodopsins inside neurons? That’s exactly what scientists were experimenting with in the early 2000s and it’s this idea that lead to the birth of optogenetics. By taking the DNA of channel rhodopsins from algae and inserting them into the membrane of neurons, scientists were able to make neurons sensitive to particular wavelengths of light. Channel rhodopsin and halorhodopsin are among the opins inserted into neurons by injecting viruses. Channel rhodopsin activates neurons while halorhodopsin silences them. Once the neuron expresses the light-gated cation channel channelrhodopsin-2 in its cells membrane, shining light on it for as little as a few milliseconds has a profound effect. It causes the opening of the channelrohodopsin-2 molecules, allowing positively charged ions to enter cell and cause the cell to fire.

Check out this video to see how optogenetics works.
Many experiments today use optogenetics to selectively turn neurons on and off in mice. What makes this method mind blowing is the high spatial and temporal resolution it gives scientists when working with the brain. It can be used on neurons in on a petri dish or within a living animal. It could be used to learn more about the function of particular brain regions. For instance, one could temporarily inactivate one region to observe how it impacts activity in other connected brain regions.
Furthermore, it’s minimally invasive: once the virus containing the rhodopsin has been injected, all the scientist needs to do is shine a pulse of light. Researchers at Stanford have used optogenetics to induce muscle contractions in mice. At Case Western Reserve University, researchers implemented it to restore motor function in rats paralyzed by spinal cord injuries. Could optogenetics be used to recover vision loss, something most humans deal with as they age? Experiments conducted on mice with a lack of photoreceptors shows that shining light on bipolar cells (containing channelrhodopsin-2) causes action potentials to fire in the visual cortex. It would be amazing if scientists could overcome biomedical and technical obstacles to make this work in humans too.
Across the river at MIT, members of the Tonegawa lab have taken the technique of optogenetics one step further. Steve Ramirez and Xu Liu have been working to localize memories in the brain and activate them with a light “switch”. And they have accomplished this feat in mice. Promising experiments with mice suggest optogenetics can be used to turn off traumatizing memories and activate pleasant once. This could have implications for PTSD, where horrific memories could be suppressed. They also have experimented with the idea of implanting false memories into the brain, which they call “Project Inception.” For more information on this work (and a good laugh), check out Steve Ramirez and Xu Liu’s TED talk.
Seems to me like optogenetics is a promising technique that can lead to breakthroughs in neuroscience.
-Srijesa K.
Sources
[1] “The Birth of Optogenetics” http://www.the-scientist.com/?articles.view/articleNo/30756/title/The-Birth-of-Optogenetics/
[2] “Potential Benefits of Optogenetics” http://optogenetics.weebly.com/what-is-it1.html
The Miracle of Neurogenesis
Neurogenesis occurs in two areas in the human adult: in the dentate gyrus of the hippocampus and in the olfactory system. The hippocampus is vital to learning new information and memory consolidation, thus it makes sense that new neurons need to be born in that region. The olfactory system is needs neurogenesis to process to new information. Majority of neurogenesis actually occurs during prenatal development. In fact humans initially have more neurons that necessary for survival. Apoptosis (programmed cell death) occurs to prune the synapses established during early development.
Many studies have been conducted that investigate ways to increase neurogenesis. Such activities include voluntary physical exercise or being in enriched environments. Experiments with rats have shown that being in an enriched environment where rats are exposed to complex objects, toys, running wheels, etc. spark improvements in performance of tasks measuring levels of learning and memory. For humans, I suppose an enriched environment could be a place involving novel stimuli or
If you think about it, how could neurogenesis be bad? I mean people with neurodegenerative diseases suffer from the consequences of neuronal loss, right? However, according to a study published in May 2014 in Science, exercise could induce amnesia. An article regarding this study states, “Adult mice that exercised on a running wheel after experiencing an event were more likely than their inactive mates to forget the experience.” Thus it appears that the neurogenesis that occurs during exercise may be “wiping out” neurons that encoded previous memories. Furthermore, when neurogenesis was pharmacologically inhibited scientists observed a recall failure in the rats. This article relates this phenomenon to the fact that children cannot form long term memories until they are 3-4 years of age.
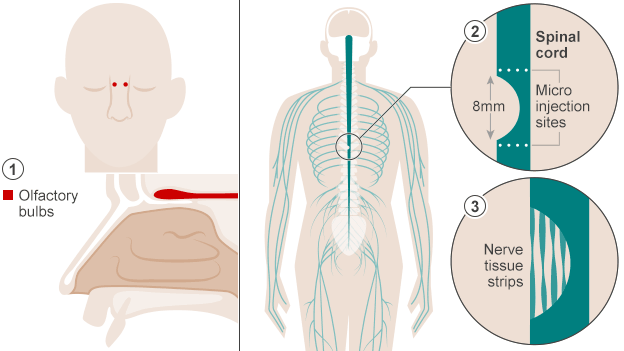
Despite controversy about neurogenesis, olfactory ensheathing cells (OECs) have been used to help a paralyzed man walk once again. An article published in October in BBC News describes how doctors in Poland accomplished this feat. The first step to this process was to extract cells from the patient’s olfactory bulbs (they removed one olfactory bulb and grew the cells in culture). Two weeks later the cells were implanted in the areas surrounding the spinal cord damage the patient had experienced. This action allowed the spinal cord cells to regenerate because the nerve grafts acted “as a bridge to cross the severed cord.” The implications of this type of surgery are pretty amazing – it could work wonders for paralyzed veterans/other individuals and people dealing with dysfunctions relevant to spinal cord damage.
Sources:
Exercise Can Erase Memories - The Scientist
Paralyzed Man Walks Again After Cell Transplant - BBC
- Srijesa K.