HiLo microscopy
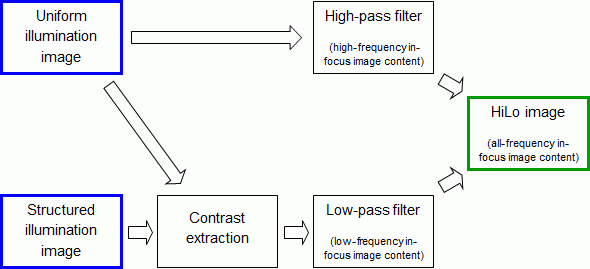
In a standard optical imaging system, an object is illuminated by a source (lamp, laser, diodes, etc.) and the resulting object signal (reflectance, fluorescence, etc.) is imaged onto a detector array (CCD camera, CMOS camera, etc.). In most cases, the illumination is uniform. An image acquired in such a manner possesses both in-focus and out-of-focus contributions. We have developed a technique, called HiLo microscopy, to reject the out-of-focus contributions.
To begin, HiLo involves acquiring a standard image with uniform illumination. As noted above, such an image contains both in-focus and out-of-focus content. By definition, out-of-focus content is blurred, and hence contains only low spatial frequency components. To reject such out-of-focus content, it suffices therefore to apply a simple high-pass filter to the image, thereby selecting only high spatial frequency components and rejecting low frequency components. Such high frequency components are thus inherently in focus. We have therefore achieved half of our goal. That is, we have extracted the high-frequency in-focus content of our standard image. The remaining task is to extract the low-frequency in-focus content.
To achieve the remaining task, we acquire a second image of our object, this time using “structured” illumination rather than uniform illumination. By structured illumination, we mean any illumination pattern that imparts spatial variations to the object signal that can be recorded by the detector array. For example, the structured illumination may be a spatially varying intensity distribution such as produced by laser speckle, fringes, a grid pattern, a checkerboard pattern, etc. Our second image is thus spatially modulated by this illumination structure. Importantly, the contrast of the imaged modulation becomes vanishingly small for object signals that arise from out of focus. That is, a measure of the local contrast of the imaged modulation provides of measure of the degree to which the object is in focus, or contains in-focus contributions. Several techniques may be used to measure the local modulation contrast (e.g. by performing local variance measurements (1), single sideband demodulation (2), double sideband demodulation, etc.). In general, these are coarse grained, meaning they provide local modulation contrast measurements with only low resolution. That is, they enable an extraction of only the low-frequency in-focus content of our image. However, by proper adjustment of the low-pass filtering provided by such coarse-grained contrast measurements, the low-frequency in-focus content derived from the structured illumination image can be made to exactly complement the high-frequency in-focus content derived from the uniform illumination image (described above). A seamless fusion of the both the high and low frequency image contents then leads to a full resolution in-focus image that contains all frequency contents within the frequency bandwidth of the imaging system.
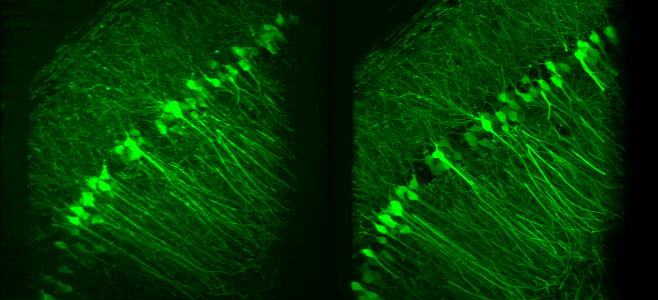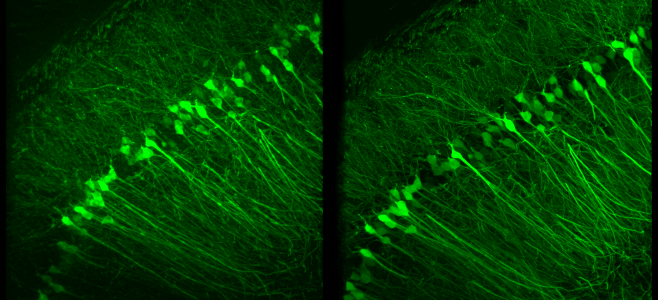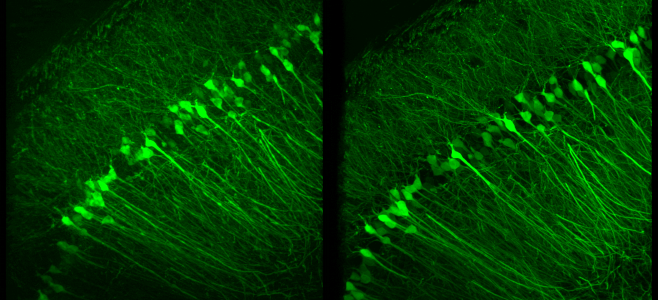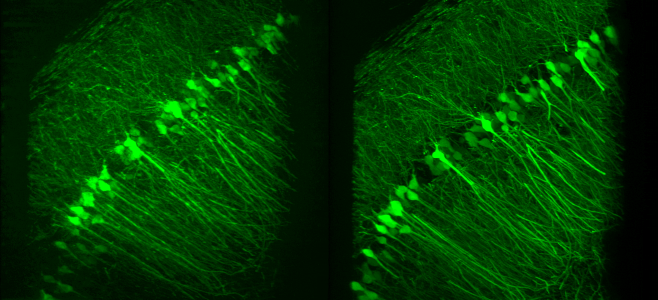
- S. Zheng, M. Koyama, J. Mertz, “Multiplane HiLo microscopy with speckle illumination and non-local means denoising”, J. Biomed. Opt. 28, 116502 (2023).
- T. N. Ford, D. Lim, and J. Mertz, “Fast optically sectioned fluorescence HiLo endomicroscopy,” J. Biomed. Opt., 17, no. 2 (2012). link
- J. Mertz, “Optical microscopy with planar or structured illumination”, Nat. Methods 8, 811-819 (2011). link
- D. Lim, T. Ford, K. K. Chu, J. Mertz, “Optically sectioned in vivo imaging with speckle illumination HiLo microscopy”, J. Biomed. Opt. 16, 016014 (2011). link
- J. Mertz, J. Kim, “Scanning light-sheet microscopy in the whole mouse brain with HiLo background rejection”, J. Biomed. Opt. 15, 016027 (2010). link
- S. Santos, K. K. Chu, D. Lim, N. Bozinovic, T. N. Ford, C. Hourtoule, A. C. Bartoo, S. K. Singh, J. Mertz, “Optically sectioned fluorescence endomicroscopy with hybrid-illumination imaging through a flexible fiber bundle”, J. Biomed. Opt. 030502 (2009). link
- N. Bozinovic, C. Ventalon, T. Ford, J. Mertz, “Fluorescence endomicroscopy with structured illumination”, Opt. Express 16, 8016-8025 (2008). link
- D. Lim, K. K. Chu, J. Mertz, “Widefield fluorescence sectioning with hybrid speckle and uniform-illumination microscopy”, Opt. Lett. 33, 1819-1821 (2008). link